Boxed Warning
WARNING: RISK OF HEPATITIS B VIRUS REACTIVATION IN PATIENTS COINFECTED WITH HCV AND HBV
Test all patients for evidence of current or prior hepatitis B virus (HBV) infection before initiating treatment with SOVALDI. HBV reactivation has been reported in HCV/HBV coinfected patients who were undergoing or had completed treatment with HCV direct acting antivirals and were not receiving HBV antiviral therapy. Some cases have resulted in fulminant hepatitis, hepatic failure, and death. Monitor HCV/HBV coinfected patients for hepatitis flare or HBV reactivation during HCV treatment and post-treatment follow-up. Initiate appropriate patient management for HBV infection as clinically indicated [see Warnings and Precautions (5.1)].
1. Indications and Usage
Adult Patients:
SOVALDI is indicated for the treatment of adult patients with chronic hepatitis C virus (HCV) infection as a component of a combination antiviral treatment regimen [see Dosage and Administration (2.2), and Clinical Studies (14)]:
- genotype 1 or 4 infection without cirrhosis or with compensated cirrhosis for use in combination with pegylated interferon and ribavirin
- genotype 2 or 3 infection without cirrhosis or with compensated cirrhosis for use in combination with ribavirin.
Pediatric Patients:
SOVALDI is indicated for the treatment of chronic HCV genotype 2 or 3 infection in pediatric patients 3 years of age and older without cirrhosis or with compensated cirrhosis for use in combination with ribavirin [see Dosage and Administration (2.3) and Clinical Studies (14.5)].
2. Dosage and Administration
2.1 Testing Prior to the Initiation of Therapy
Test all patients for evidence of current or prior HBV infection by measuring hepatitis B surface antigen (HBsAg) and hepatitis B core antibody (anti-HBc) before initiating HCV treatment with SOVALDI [see Warnings and Precautions (5.1)] .
2.2 Recommended Dosage in Adults
The recommended dosage of SOVALDI is one 400 mg tablet, taken orally, once daily with or without food [see Clinical Pharmacology (12.3)].
Administer SOVALDI in combination with ribavirin or in combination with pegylated interferon and ribavirin for the treatment of HCV. The recommended treatment regimen and duration for SOVALDI combination therapy is provided in Table 1.
For patients with HCV/HIV-1 coinfection, follow the dosage recommendations in Table 1. Refer to Drug Interactions (7) for dosage recommendations for concomitant HIV-1 antiviral drugs.
| Patient Population | Treatment Regimen and Duration | |
|---|---|---|
| Genotype 1 or 4 | Treatment-naïve without cirrhosis or with compensated cirrhosis (Child-Pugh A) | SOVALDI + peginterferon alfa* + ribavirin† 12 weeks |
| Genotype 2 | Treatment-naïve and treatment-experienced‡ without cirrhosis or with compensated cirrhosis (Child-Pugh A) | SOVALDI + ribavirin† 12 weeks |
| Genotype 3 | Treatment-naïve and treatment-experienced‡ without cirrhosis or with compensated cirrhosis (Child-Pugh A) | SOVALDI + ribavirin† 24 weeks |
| ||
Patients with Genotype 1 HCV Who are Ineligible to Receive an Interferon-Based Regimen
SOVALDI in combination with ribavirin for 24 weeks can be considered as a therapeutic option for patients with genotype 1 infection who are ineligible to receive an interferon-based regimen [see Clinical Studies (14.4)]. Treatment decision should be guided by an assessment of the potential benefits and risks for the individual patient.
Patients with Hepatocellular Carcinoma Awaiting Liver Transplantation
Administer SOVALDI in combination with ribavirin for up to 48 weeks or until the time of liver transplantation, whichever occurs first, to prevent post-transplant HCV reinfection [see Use in Specific Populations (8.8)].
2.3 Recommended Dosage in Pediatric Patients 3 Years of Age and Older with Genotype 2 or 3 HCV
The recommended treatment regimen, duration, and recommended dosage for SOVALDI combination therapy is provided in Table 2 and Table 3. Table 4 provides the weight-based dosage of ribavirin when used in combination with SOVALDI for pediatric patients. For patients with HCV/HIV-1 coinfection, follow the dosage recommendations in Table 3 and Table 4. Refer to Drug Interactions (7) for dosage recommendations for concomitant HIV-1 antiviral drugs. In pediatric patients with hepatocellular carcinoma awaiting liver transplantation, administer SOVALDI in combination with ribavirin for up to 48 weeks or until the time of liver transplantation, whichever occurs first, to prevent post-transplant HCV reinfection [see Use in Specific Populations (8.8)].
| Patient Population | Treatment Regimen and Duration | |
|---|---|---|
| Genotype 2 | Treatment-naïve and treatment-experienced* without cirrhosis or with compensated cirrhosis (Child-Pugh A) | SOVALDI + ribavirin† 12 weeks |
| Genotype 3 | Treatment-naïve and treatment-experienced* without cirrhosis or with compensated cirrhosis (Child-Pugh A) | SOVALDI + ribavirin† 24 weeks |
| ||
The recommended dosage of SOVALDI in pediatric patients 3 years and older with genotype 2 or 3 HCV using SOVALDI tablets or oral pellets (with or without food) is based on weight (Table 3), and is to be taken orally once daily in combination with ribavirin [see Dosage and Administration (2.4), Use in Specific Populations (8.4), Clinical Pharmacology (12.3), and Clinical Studies (14.5)]. SOVALDI pellets can be taken by pediatric patients who cannot swallow the tablet formulation [see Dosage and Administration (2.4)].
| Body Weight (kg) | Dosing of SOVALDI Tablets or Oral Pellets | SOVALDI Daily Dose |
|---|---|---|
| at least 35 | one 400 mg tablet once daily or two 200 mg tablets once daily or two 200 mg packets of pellets once daily | 400 mg per day |
| 17 to less than 35 | one 200 mg tablet once daily or one 200 mg packet of pellets once daily | 200 mg per day |
| less than 17 | one 150 mg packet of pellets once daily | 150 mg per day |
| Body Weight (kg) | Oral Ribavirin Daily Dosage* |
|---|---|
| less than 47 | 15 mg per kg per day (divided dose AM and PM) |
| 47–49 | 600 mg per day (1 × 200 mg AM, 2 × 200 mg PM) |
| 50–65 | 800 mg per day (2 × 200 mg AM, 2 × 200 mg PM) |
| 66–80 | 1000 mg per day (2 × 200 mg AM, 3 × 200 mg PM) |
| greater than 80 | 1200 mg per day (3 × 200 mg AM, 3 × 200 mg PM) |
| |
2.4 Preparation and Administration of Oral Pellets
See the SOVALDI oral pellets full Instructions for Use for details on the preparation and administration of SOVALDI pellets.
Do not chew SOVALDI pellets. If SOVALDI pellets are administered with food, sprinkle the pellets on one or more spoonfuls of non-acidic soft food at or below room temperature. Examples of non-acidic foods include pudding, chocolate syrup, mashed potato, and ice cream. Take SOVALDI pellets within 30 minutes of gently mixing with food and swallow the entire contents without chewing to avoid a bitter aftertaste.
2.5 Dosage Modification
Dosage reduction of SOVALDI is not recommended.
If a patient has a serious adverse reaction potentially related to peginterferon alfa and/or ribavirin, the peginterferon alfa and/or ribavirin dosage should be reduced or discontinued, if appropriate, until the adverse reaction abates or decreases in severity. Refer to the peginterferon alfa and ribavirin prescribing information for additional information about how to reduce and/or discontinue the peginterferon alfa and/or ribavirin dosage.
2.6 Discontinuation of Dosing
If the other agents used in combination with SOVALDI are permanently discontinued, SOVALDI should also be discontinued.
2.7 Severe Renal Impairment and End Stage Renal Disease
No dosage recommendation can be given for patients with severe renal impairment (estimated Glomerular Filtration Rate [eGFR] less than 30 mL/min/1.73m2) or with end stage renal disease (ESRD) due to higher exposures (up to 20-fold) of the predominant sofosbuvir metabolite [see Use in Specific Populations (8.6) and Clinical Pharmacology (12.3)].
3. Dosage Forms and Strengths
SOVALDI is available as tablets or pellets for oral use. Each dosage form is available in two dose strengths.
- 400 mg Tablets: 400 mg sofosbuvir: yellow, capsule-shaped, film-coated tablet debossed with "GSI" on one side and "7977" on the other side.
- 200 mg Tablets: 200 mg sofosbuvir: yellow, oval-shaped, film-coated tablet debossed with "GSI" on one side and "200" on the other side.
- 200 mg Pellets: 200 mg sofosbuvir: white to off-white pellets in unit-dose packets.
- 150 mg Pellets: 150 mg sofosbuvir: white to off-white pellets in unit-dose packets.
4. Contraindications
5. Warnings and Precautions
5.1 Risk of Hepatitis B Virus Reactivation in Patients Coinfected with HCV and HBV
Hepatitis B virus (HBV) reactivation has been reported in HCV/HBV coinfected patients who were undergoing or had completed treatment with HCV direct acting antivirals, and who were not receiving HBV antiviral therapy. Some cases have resulted in fulminant hepatitis, hepatic failure, and death. Cases have been reported in patients who are HBsAg positive and also in patients with serologic evidence of resolved HBV infection (i.e., HBsAg negative and anti-HBc positive). HBV reactivation has also been reported in patients receiving certain immunosuppressant or chemotherapeutic agents; the risk of HBV reactivation associated with treatment with HCV direct-acting antivirals may be increased in these patients.
HBV reactivation is characterized as an abrupt increase in HBV replication manifesting as a rapid increase in serum HBV DNA level. In patients with resolved HBV infection, reappearance of HBsAg can occur. Reactivation of HBV replication may be accompanied by hepatitis, i.e., increases in aminotransferase levels and, in severe cases, increases in bilirubin levels, liver failure, and death can occur.
Test all patients for evidence of current or prior HBV infection by measuring HBsAg and anti-HBc before initiating HCV treatment with SOVALDI. In patients with serologic evidence of HBV infection, monitor for clinical and laboratory signs of hepatitis flare or HBV reactivation during HCV treatment with SOVALDI and during post-treatment follow-up. Initiate appropriate patient management for HBV infection as clinically indicated.
5.2 Serious Symptomatic Bradycardia When Coadministered with Amiodarone
Postmarketing cases of symptomatic bradycardia and cases requiring pacemaker intervention have been reported when amiodarone is coadministered with a sofosbuvir-containing regimen. A fatal cardiac arrest was reported in a patient taking amiodarone who was coadministered a sofosbuvir-containing regimen (HARVONI [ledipasvir/sofosbuvir]). Bradycardia has generally occurred within hours to days, but cases have been observed up to 2 weeks after initiating HCV treatment. Patients also taking beta blockers, or those with underlying cardiac comorbidities and/or advanced liver disease may be at increased risk for symptomatic bradycardia with coadministration of amiodarone. Bradycardia generally resolved after discontinuation of HCV treatment. The mechanism for this effect is unknown.
Coadministration of amiodarone with SOVALDI is not recommended. For patients taking amiodarone who have no other alternative, viable treatment options and who will be coadministered SOVALDI:
- Counsel patients about the risk of serious symptomatic bradycardia
- Cardiac monitoring in an in-patient setting for the first 48 hours of coadministration is recommended, after which outpatient or self-monitoring of the heart rate should occur on a daily basis through at least the first 2 weeks of treatment.
Patients who are taking SOVALDI who need to start amiodarone therapy due to no other alternative, viable treatment options should undergo similar cardiac monitoring as outlined above.
Due to amiodarone's long half-life, patients discontinuing amiodarone just prior to starting SOVALDI should also undergo similar cardiac monitoring as outlined above.
Patients who develop signs or symptoms of bradycardia should seek medical evaluation immediately. Symptoms may include near-fainting or fainting, dizziness or lightheadedness, malaise, weakness, excessive tiredness, shortness of breath, chest pains, confusion or memory problems [see Adverse Reactions (6.2), Drug Interactions (7.1)].
5.3 Risk of Reduced Therapeutic Effect Due to Use with P-gp Inducers
Drugs that are P-gp inducers in the intestine (e.g., rifampin, St. John's wort) may significantly decrease sofosbuvir plasma concentrations and may lead to a reduced therapeutic effect of SOVALDI. The use of rifampin and St. John's wort with SOVALDI is not recommended [see Drug Interactions (7.1)].
5.4 Risks Associated with Combination Treatment
Because SOVALDI is used in combination with other antiviral drugs for treatment of HCV infection, consult the prescribing information for these drugs used in combination with SOVALDI. Warnings and Precautions related to these drugs also apply to their use in SOVALDI combination treatment.
6. Adverse Reactions
The following serious adverse reactions are described below and elsewhere in the labeling:
- Serious Symptomatic Bradycardia When Coadministered with Amiodarone [see Warnings and Precautions (5.2)].
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
When SOVALDI is administered with ribavirin or peginterferon alfa/ribavirin, refer to the respective prescribing information for a description of adverse reactions associated with their use.
Adverse Reactions in Adult Subjects
The safety assessment of SOVALDI was based on pooled Phase 3 clinical trial data (both controlled and uncontrolled) including:
- 650 subjects who received SOVALDI + ribavirin (RBV) combination therapy for 12 weeks,
- 98 subjects who received SOVALDI + ribavirin combination therapy for 16 weeks,
- 250 subjects who received SOVALDI + ribavirin combination therapy for 24 weeks,
- 327 subjects who received SOVALDI + peginterferon (Peg-IFN) alfa + ribavirin combination therapy for 12 weeks,
- 243 subjects who received peginterferon alfa + ribavirin for 24 weeks, and
- 71 subjects who received placebo (PBO) for 12 weeks [see Clinical Studies (14)].
The proportion of subjects who permanently discontinued treatment due to adverse events was 4% for subjects receiving placebo, 1% for subjects receiving SOVALDI + ribavirin for 12 weeks, less than 1% for subjects receiving SOVALDI + ribavirin for 24 weeks, 11% for subjects receiving peginterferon alfa + ribavirin for 24 weeks and 2% for subjects receiving SOVALDI + peginterferon alfa + ribavirin for 12 weeks.
Adverse events observed in at least 15% of subjects in the Phase 3 clinical trials outlined above are provided in Table 5. A side-by-side tabulation is displayed to simplify presentation; direct comparison across trials should not be made due to differing trial designs.
The most common adverse events (at least 20%) for SOVALDI + ribavirin combination therapy were fatigue and headache. The most common adverse events (at least 20%) for SOVALDI + peginterferon alfa + ribavirin combination therapy were fatigue, headache, nausea, insomnia and anemia.
| Interferon-free Regimens | Interferon-containing Regimens | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PBO 12 weeks | SOVALDI + RBV*
12 weeks | SOVALDI + RBV*
24 weeks | Peg-IFN alfa + RBV†
24 weeks | SOVALDI + Peg-IFN alfa + RBV*
12 weeks | |||||||||
| N=71 | N=650 | N=250 | N=243 | N=327 | |||||||||
| Fatigue | 24% | 38% | 30% | 55% | 59% | ||||||||
| Headache | 20% | 24% | 30% | 44% | 36% | ||||||||
| Nausea | 18% | 22% | 13% | 29% | 34% | ||||||||
| Insomnia | 4% | 15% | 16% | 29% | 25% | ||||||||
| Pruritus | 8% | 11% | 27% | 17% | 17% | ||||||||
| Anemia | 0% | 10% | 6% | 12% | 21% | ||||||||
| Asthenia | 3% | 6% | 21% | 3% | 5% | ||||||||
| Rash | 8% | 8% | 9% | 18% | 18% | ||||||||
| Decreased Appetite | 10% | 6% | 6% | 18% | 18% | ||||||||
| Chills | 1% | 2% | 2% | 18% | 17% | ||||||||
| Influenza Like Illness | 3% | 3% | 6% | 18% | 16% | ||||||||
| Pyrexia | 0% | 4% | 4% | 14% | 18% | ||||||||
| Diarrhea | 6% | 9% | 12% | 17% | 12% | ||||||||
| Neutropenia | 0% | <1% | <1% | 12% | 17% | ||||||||
| Myalgia | 0% | 6% | 9% | 16% | 14% | ||||||||
| Irritability | 1% | 10% | 10% | 16% | 13% | ||||||||
| |||||||||||||
With the exception of anemia and neutropenia, the majority of events presented in Table 5 occurred at severity of grade 1 in SOVALDI-containing regimens.
Less Common Adverse Reactions Reported in Clinical Trials (less than 1%): The following adverse reactions occurred in less than 1% of subjects receiving SOVALDI in a combination regimen in any one trial. These events have been included because of their seriousness or assessment of potential causal relationship.
Hematologic Effects: pancytopenia (particularly in subjects receiving concomitant pegylated interferon).
Psychiatric Disorders: severe depression (particularly in subjects with pre-existing history of psychiatric illness), including suicidal ideation and suicide.
Laboratory Abnormalities:
Changes in selected hematological parameters are described in Table 6. A side-by-side tabulation is displayed to simplify presentation; direct comparison across trials should not be made due to differing trial designs.
| Hematological Parameters | Interferon-free Regimens | Interferon-containing Regimens | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PBO 12 weeks | SOVALDI + RBV*
12 weeks | SOVALDI + RBV*
24 weeks | Peg-IFN + RBV†
24 weeks | SOVALDI + Peg-IFN + RBV*
12 weeks | |||||||||
| N=71 | N=647 | N=250 | N=242 | N=327 | |||||||||
| Hemoglobin (g/dL) | |||||||||||||
| <10 | 0 | 8% | 6% | 14% | 23% | ||||||||
| <8.5 | 0 | 1% | <1% | 2% | 2% | ||||||||
| Neutrophils (×109/L) | |||||||||||||
| ≥0.5 – <0.75 | 1% | <1% | 0 | 12% | 15% | ||||||||
| <0.5 | 0 | <1% | 0 | 2% | 5% | ||||||||
| Platelets (×109/L) | |||||||||||||
| ≥25 – <50 | 3% | <1% | 1% | 7% | <1% | ||||||||
| <25 | 0 | 0 | 0 | 0 | 0 | ||||||||
| |||||||||||||
Bilirubin Elevations
Total bilirubin elevation of more than 2.5×ULN was observed in none of the subjects in the SOVALDI + peginterferon alfa + ribavirin 12 weeks group and in 1%, 3% and 3% of subjects in the peginterferon alfa + ribavirin 24 weeks, SOVALDI + ribavirin 12 weeks and SOVALDI + ribavirin 24 weeks groups, respectively. Bilirubin levels peaked during the first 1 to 2 weeks of treatment and subsequently decreased and returned to baseline levels by post-treatment Week 4. These bilirubin elevations were not associated with transaminase elevations.
Creatine Kinase Elevations
Creatine kinase was assessed in the FISSION and NEUTRINO trials. Isolated, asymptomatic creatine kinase elevation of greater than or equal to 10×ULN was observed in less than 1%, 1% and 2% of subjects in the peginterferon alfa + ribavirin 24 weeks, SOVALDI + peginterferon alfa + ribavirin 12 weeks and SOVALDI + ribavirin 12 weeks groups, respectively.
Lipase Elevations
Isolated, asymptomatic lipase elevation of greater than 3×ULN was observed in less than 1%, 2%, 2%, and 2% of subjects in the SOVALDI + peginterferon alfa + ribavirin 12 weeks, SOVALDI + ribavirin 12 weeks, SOVALDI + ribavirin 24 weeks and peginterferon alfa + ribavirin 24 weeks groups, respectively.
Patients with HCV/HIV-1 Coinfection
SOVALDI used in combination with ribavirin was assessed in 223 HCV/HIV-1 coinfected subjects [see Clinical Studies (14.4)]. The safety profile in HCV/HIV-1 coinfected subjects was similar to that observed in HCV mono-infected subjects. Elevated total bilirubin (grade 3 or 4) was observed in 30/32 (94%) subjects receiving atazanavir as part of the antiretroviral regimen. None of the subjects had concomitant transaminase increases. Among subjects not taking atazanavir, grade 3 or 4 elevated total bilirubin was observed in 2 (1.5%) subjects, similar to the rate observed with HCV mono-infected subjects receiving SOVALDI + ribavirin in Phase 3 trials.
Adverse Reactions in Pediatric Subjects 3 Years of Age and Older
The safety assessment of SOVALDI in pediatric subjects 3 years of age and older is based on data from 106 subjects who were treated with SOVALDI plus ribavirin for 12 weeks (genotype 2 subjects) or 24 weeks (genotype 3 subjects) in a Phase 2, open-label clinical trial. The adverse reactions observed were consistent with those observed in clinical studies of SOVALDI plus ribavirin in adults. Among pediatric subjects 3 years to < 12 years of age taking SOVALDI in combination with ribavirin oral solution, decreased appetite was observed in 13% (7/54) of subjects [see Clinical Studies 14.5)].
In a 5-year follow-up study, 88 of the 106 subjects from the Phase 2 open-label clinical trial (Study 1112) were followed for a median (Q1, Q3) duration of 239 (179, 244) weeks. No notable effects on growth as assessed by changes from baseline through end of study were observed for height, weight, BMI percentiles, and Z-scores for any age group. No notable effects were observed on the development of secondary sexual characteristics of subjects as assessed by changes from baseline through end of study in Tanner pubertal stages [see Use in Specific Populations (8.4)].
6.2 Postmarketing Experience
The following adverse reactions have been identified during post approval use of SOVALDI. Because postmarketing reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Cardiac Disorders
Serious symptomatic bradycardia has been reported in patients taking amiodarone who initiate treatment with a sofosbuvir-containing regimen [see Warnings and Precautions (5.2), Drug Interactions (7.1)].
7. Drug Interactions
7.1 Potentially Significant Drug Interactions
Sofosbuvir is a substrate of drug transporter P-gp and breast cancer resistance protein (BCRP) while the predominant circulating metabolite GS-331007 is not. Drugs that are P-gp inducers in the intestine (e.g., rifampin or St. John's wort) may decrease sofosbuvir plasma concentration, leading to reduced therapeutic effect of SOVALDI, and thus concomitant use with SOVALDI is not recommended [see Warnings and Precautions (5.3)].
Clearance of HCV infection with direct acting antivirals may lead to changes in hepatic function, which may impact the safe and effective use of concomitant medications. For example, altered blood glucose control resulting in serious symptomatic hypoglycemia has been reported in diabetic patients in postmarketing case reports and published epidemiological studies. Management of hypoglycemia in these cases required either discontinuation or dose modification of concomitant medications used for diabetes treatment.
Frequent monitoring of relevant laboratory parameters (e.g. International Normalized Ratio [INR] in patients taking warfarin, blood glucose levels in diabetic patients) or drug concentrations of concomitant medications such as cytochrome P450 substrates with a narrow therapeutic index (e.g. certain immunosuppressants) is recommended to ensure safe and effective use. Dose adjustments of concomitant medications may be necessary.
Information on potential drug interactions with SOVALDI is summarized in Table 7. The table is not all-inclusive [see Warnings and Precautions (5.2, 5.3) and Clinical Pharmacology (12.3)].
| Concomitant Drug Class: Drug Name | Effect on Concentration† | Clinical Comment |
|---|---|---|
| Antiarrhythmics: amiodarone | Effect on amiodarone and sofosbuvir concentrations unknown | Coadministration of amiodarone with a sofosbuvir-containing regimen may result in serious symptomatic bradycardia. The mechanism of this effect is unknown. Coadministration of amiodarone with SOVALDI is not recommended; if coadministration is required, cardiac monitoring is recommended [see Warnings and Precautions (5.2), Adverse Reactions (6.2)]. |
| Anticonvulsants: Carbamazepine phenytoin phenobarbital oxcarbazepine | ↓ sofosbuvir ↓ GS-331007 | Coadministration of SOVALDI with carbamazepine, phenytoin, phenobarbital or oxcarbazepine is expected to decrease the concentration of sofosbuvir, leading to reduced therapeutic effect of SOVALDI. Coadministration is not recommended. |
| Antimycobacterials: Rifabutin rifampin rifapentine | ↓ sofosbuvir ↓ GS-331007 | Coadministration of SOVALDI with rifabutin or rifapentine is expected to decrease the concentration of sofosbuvir, leading to reduced therapeutic effect of SOVALDI. Coadministration is not recommended. Coadministration of SOVALDI with rifampin, an intestinal P-gp inducer, is not recommended [see Warnings and Precautions (5.3)]. |
| Herbal Supplements: St. John's wort (Hypericum perforatum) | ↓ sofosbuvir ↓ GS-331007 | Coadministration of SOVALDI with St. John's wort, an intestinal P-gp inducer, is not recommended [see Warnings and Precautions (5.3)]. |
| HIV Protease Inhibitors: tipranavir/ritonavir | ↓ sofosbuvir ↓ GS-331007 | Coadministration of SOVALDI with tipranavir/ritonavir is expected to decrease the concentration of sofosbuvir, leading to reduced therapeutic effect of SOVALDI. Coadministration is not recommended. |
| ||
7.2 Drugs without Clinically Significant Interactions with SOVALDI
Based on drug interaction studies conducted with SOVALDI, no clinically significant drug interactions have been either observed or are expected when SOVALDI is combined with the following drugs [see Clinical Pharmacology (12.3)]: cyclosporine, darunavir/ritonavir, efavirenz, emtricitabine, methadone, oral contraceptives, raltegravir, rilpivirine, tacrolimus, or tenofovir disoproxil fumarate.
8. Use in Specific Populations
8.1 Pregnancy
Risk Summary
If SOVALDI is administered with ribavirin or peginterferon alfa and ribavirin, the combination regimen is contraindicated in pregnant women and in men whose female partners are pregnant. Refer to the ribavirin and/or peginterferon alfa prescribing information for more information on ribavirin- and peginterferon alfa-associated risks of use during pregnancy.
No adequate human data are available to establish whether or not SOVALDI poses a risk to pregnancy outcomes. In animal reproduction studies, no evidence of adverse developmental outcomes was observed with sofosbuvir at exposures greater than those in humans at the recommended human dose (RHD) [see Data]. During organogenesis in the rat and rabbit, systemic exposures (AUC) to the predominant circulating metabolite of sofosbuvir (GS-331007) were ≥5 (rats) and 12 (rabbits) times the exposure in humans at the RHD. In the rat pre/postnatal development study, maternal systemic exposure (AUC) to GS-331007 was ≥6 times the exposure in humans at the RHD.
The background risk of major birth defects and miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2–4% and 15–20%, respectively.
Data
Animal Data
Sofosbuvir was administered orally to pregnant rats (up to 500 mg/kg/day) and rabbits (up to 300 mg/kg/day) on gestation days 6 to 18 and 6 to 19, respectively, and also to rats (oral doses up to 500 mg/kg/day) on gestation day 6 to lactation/post-partum day 20. No significant effects on embryo-fetal (rats and rabbits) or pre/postnatal (rats) development were observed at the highest doses tested. Systemic exposures (AUC) to the predominant circulating metabolite of sofosbuvir (GS-331007) were ≥5 (rats) and 12 (rabbits) times the exposure in humans at the RHD, with exposures increasing during gestation from approximately 5 to 10 (rats) and 12 to 28 (rabbits) times the exposure in humans at the RHD.
8.2 Lactation
Risk Summary
It is not known whether sofosbuvir or its metabolites are present in human breast milk, affect human milk production or have effects on the breastfed infant. The predominant circulating metabolite of sofosbuvir (GS-331007) was the primary component observed in the milk of lactating rats, without effect on nursing pups [see Data].
The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for SOVALDI and any potential adverse effects on the breastfed child from SOVALDI or from the underlying maternal condition.
If SOVALDI is administered with ribavirin, the nursing mother's information for ribavirin also applies to this combination regimen. Refer to the ribavirin prescribing information for more information on use during lactation.
Data
Animal Data
No effects of sofosbuvir on growth and postnatal development were observed in nursing pups at the highest dose tested in rats. Maternal systemic exposure (AUC) to the predominant circulating metabolite of sofosbuvir (GS-331007) was approximately 12 times the exposure in humans at the RHD, with exposure of approximately 2% that of maternal exposure observed in nursing pups on lactation day 10. In a lactation study, sofosbuvir metabolites (primarily GS-331007) were excreted into the milk of lactating rats following administration of a single oral dose of sofosbuvir (20 mg/kg) on lactation day 2, with milk concentrations of approximately 10% that of maternal plasma concentrations observed 1 hour post-dose.
8.3 Females and Males of Reproductive Potential
If SOVALDI is administered with ribavirin or peginterferon and ribavirin, the information for ribavirin and peginterferon with regard to pregnancy testing, contraception, and infertility also applies to these combination regimens. Refer to ribavirin and/or peginterferon prescribing information for additional information.
8.4 Pediatric Use
The safety, pharmacokinetics, and efficacy of SOVALDI in pediatric patients 3 years of age and older with genotype 2 and 3 infection have been established. SOVALDI was evaluated in an open-label clinical trial (Study 1112), which included 106 subjects (31 genotype 2; 75 genotype 3) 3 years of age and older. The safety, pharmacokinetics, and efficacy were comparable to that observed in adults [see Dosage and Administration (2.3), Adverse Reactions (6.1), Clinical Pharmacology (12.3), and Clinical Studies (14.5)].
The safety and efficacy of SOVALDI in pediatric patients 3 years of age and older with compensated cirrhosis is supported by comparable sofosbuvir and GS-331007 exposures between: 1) adults and pediatric patients without cirrhosis and 2) adults without cirrhosis and adults with compensated cirrhosis. Thus, similar efficacy would be expected for pediatric patients with compensated cirrhosis as adults with compensated cirrhosis.
The safety and efficacy of SOVALDI have not been established in pediatric patients less than 3 years of age with HCV genotype 2 or 3. The safety and efficacy of SOVALDI have not been established in pediatric patients with HCV genotype 1 or 4.
In a 5-year follow-up study, the long-term effects of SOVALDI on pediatric growth were assessed in 88 pediatric subjects 3 years of age and older treated with SOVALDI in Study 1112. No notable effects on growth from baseline through end of study were observed [see Adverse Reactions (6.1)]. All subjects who had achieved SVR12 maintained SVR through end of study.
8.5 Geriatric Use
SOVALDI was administered to 90 subjects aged 65 and over. The response rates observed for subjects over 65 years of age were similar to that of younger subjects across treatment groups. No dosage adjustment of SOVALDI is warranted in geriatric patients [see Clinical Pharmacology (12.3)].
8.6 Renal Impairment
No dosage adjustment of SOVALDI is required for patients with mild or moderate renal impairment. The safety and efficacy of SOVALDI have not been established in patients with severe renal impairment (eGFR less than 30 mL/min/1.73m2) or ESRD requiring hemodialysis. No dosage recommendation can be given for patients with severe renal impairment or ESRD [see Dosage and Administration (2.7) and Clinical Pharmacology (12.3)]. Refer also to ribavirin and peginterferon alfa prescribing information for patients with CrCl less than 50 mL/min.
8.7 Hepatic Impairment
No dosage adjustment of SOVALDI is required for patients with mild, moderate or severe hepatic impairment (Child-Pugh Class A, B or C) [see Clinical Pharmacology (12.3)]. Safety and efficacy of SOVALDI have not been established in patients with decompensated cirrhosis. See peginterferon alfa prescribing information for contraindication in hepatic decompensation.
8.8 Patients with Hepatocellular Carcinoma Awaiting Liver Transplantation
SOVALDI was studied in HCV-infected adult subjects with hepatocellular carcinoma prior to undergoing liver transplantation in an open-label clinical trial evaluating the safety and efficacy of SOVALDI and ribavirin administered pre-transplant to prevent post-transplant HCV reinfection. The primary endpoint of the trial was post-transplant virologic response (pTVR) defined as HCV RNA less than lower limit of quantification (LLOQ) at 12 weeks post-transplant. HCV-infected subjects, regardless of genotype, with hepatocellular carcinoma (HCC) meeting the MILAN criteria (defined as the presence of a tumor 5 cm or less in diameter in patients with single hepatocellular carcinomas and no more than three tumor nodules, each 3 cm or less in diameter in patients with multiple tumors and no extrahepatic manifestations of the cancer or evidence of vascular invasion of tumor) received 400 mg SOVALDI and weight-based 1000–1200 mg ribavirin daily for 24–48 weeks or until the time of liver transplantation, whichever occurred first. An interim analysis was conducted on 61 subjects who received SOVALDI and ribavirin; 45 subjects had HCV genotype 1; 44 subjects had a baseline CPT score less than 7 and all subjects had a baseline unadjusted MELD score up to 14. Of these 61 subjects, 41 subjects underwent liver transplantation following up to 48 weeks of treatment with SOVALDI and ribavirin; 37 had HCV RNA less than LLOQ at the time of transplantation. Of the 37 subjects, the post-transplant virologic response (pTVR) rate is 64% (23/36) in the 36 evaluable subjects who have reached the 12 week post-transplant time point. The safety profile of SOVALDI and ribavirin in HCV-infected subjects prior to liver transplantation was comparable to that observed in subjects treated with SOVALDI and ribavirin in Phase 3 clinical trials.
10. Overdosage
The highest documented dosage of sofosbuvir was a single dose of sofosbuvir 1200 mg (three times the recommended dosage) administered to 59 healthy subjects. In that trial, there were no untoward effects observed at this dosage level, and adverse events were similar in frequency and severity to those reported in the placebo and sofosbuvir 400 mg treatment groups. The effects of higher dosages are not known.
No specific antidote is available for overdose with SOVALDI. If overdose occurs, the patient must be monitored for evidence of toxicity. Treatment of overdose with SOVALDI consists of general supportive measures including monitoring of vital signs as well as observation of the clinical status of the patient. A 4-hour hemodialysis session removed 18% of the administered dose.
11. Description
SOVALDI (sofosbuvir) is a nucleotide analog inhibitor of HCV NS5B polymerase.
The IUPAC name for sofosbuvir is (S)-isopropyl 2-((S)-(((2R,3R,4R,5R)-5-(2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl)-4-fluoro-3-hydroxy-4-methyltetrahydrofuran-2-yl)methoxy)-(phenoxy)phosphorylamino)propanoate. It has a molecular formula of C22H29FN3O9P and a molecular weight of 529.45. It has the following structural formula:
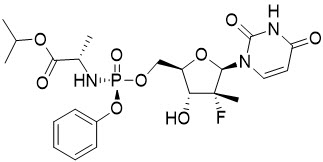
Sofosbuvir is a white to off-white crystalline solid with a solubility of ≥ 2 mg/mL across the pH range of 2–7.7 at 37 °C and is slightly soluble in water.
SOVALDI tablets, 200 mg or 400 mg, are for oral administration. Each tablet contains 200 mg or 400 mg of sofosbuvir. The tablets include the following inactive ingredients: colloidal silicon dioxide, croscarmellose sodium, magnesium stearate, mannitol, and microcrystalline cellulose. The tablets are film-coated with a coating material containing the following inactive ingredients: polyethylene glycol, polyvinyl alcohol, talc, titanium dioxide, and yellow iron oxide.
SOVALDI pellets, 150 mg or 200 mg, are for oral administration, supplied as white to off-white pellets in unit-dose packets. Each unit-dose packet contains 150 mg or 200 mg of sofosbuvir. The pellets include the following inactive ingredients: amino methacrylate copolymer, colloidal silicon dioxide, croscarmellose sodium, hydroxypropyl cellulose, hypromellose, lactose monohydrate, microcrystalline cellulose, polyethylene glycol, silicon dioxide, sodium lauryl sulfate, sodium stearyl fumarate, stearic acid, and talc.
12. Clinical Pharmacology
12.1 Mechanism of Action
Sofosbuvir is a direct-acting antiviral agent against the hepatitis C virus [see Microbiology (12.4)].
12.2 Pharmacodynamics
Cardiac Electrophysiology
The effect of sofosbuvir 400 and 1200 mg (three times the recommended dosage) on QTc interval was evaluated in a randomized, single-dose, placebo- and active-controlled (moxifloxacin 400 mg) four period crossover thorough QT trial in 59 healthy subjects. At a dosage three times the maximum recommended dosage, SOVALDI does not prolong QTc to any clinically relevant extent.
12.3 Pharmacokinetics
Absorption
The pharmacokinetic properties of sofosbuvir and the predominant circulating metabolite GS-331007 have been evaluated in healthy adult subjects and in subjects with chronic hepatitis C. Following oral administration of SOVALDI, sofosbuvir was absorbed with a peak plasma concentration observed at ~0.5–2 hour post-dose, regardless of dose level. Peak plasma concentration of GS-331007 was observed between 2 to 4 hours post-dose. Based on population pharmacokinetic analysis in subjects with genotype 1 to 6 HCV infection who were coadministered ribavirin (with or without pegylated interferon), geometric mean steady state AUC0–24 was 969 ng∙hr/mL for sofosbuvir (N=838), and 6790 ng∙hr/mL for GS-331007 (N=1695). Relative to healthy subjects administered sofosbuvir alone (N=272), the sofosbuvir AUC0–24 was 60% higher; and GS-331007 AUC0–24 was 39% lower, respectively, in HCV-infected subjects. Sofosbuvir and GS-331007 AUCs are near dose proportional over the dose range of 200 mg to 1200 mg.
Effect of Food
Relative to fasting conditions, the administration of a single dose of SOVALDI with a standardized high fat meal did not substantially affect the sofosbuvir Cmax or AUC0–inf. The exposure of GS-331007 was not altered in the presence of a high-fat meal. Therefore, SOVALDI can be administered without regard to food.
Distribution
Sofosbuvir is approximately 61–65% bound to human plasma proteins and the binding is independent of drug concentration over the range of 1 microgram/mL to 20 microgram/mL. Protein binding of GS-331007 was minimal in human plasma. After a single 400 mg dose of [14C]-sofosbuvir in healthy subjects, the blood to plasma ratio of 14C-radioactivity was approximately 0.7.
Metabolism
Sofosbuvir is extensively metabolized in the liver to form the pharmacologically active nucleoside analog triphosphate GS-461203. The metabolic activation pathway involves sequential hydrolysis of the carboxyl ester moiety catalyzed by human cathepsin A (CatA) or carboxylesterase 1 (CES1) and phosphoramidate cleavage by histidine triad nucleotide-binding protein 1 (HINT1) followed by phosphorylation by the pyrimidine nucleotide biosynthesis pathway. Dephosphorylation results in the formation of nucleoside metabolite GS-331007 that cannot be efficiently rephosphorylated and lacks anti-HCV activity in vitro.
After a single 400 mg oral dose of [14C]-sofosbuvir, sofosbuvir and GS-331007 accounted for approximately 4% and greater than 90% of drug related material (sum of molecular weight-adjusted AUC of sofosbuvir and its metabolites) systemic exposure, respectively.
Elimination
Following a single 400 mg oral dose of [14C]-sofosbuvir, mean total recovery of the dose was greater than 92%, consisting of approximately 80%, 14%, and 2.5% recovered in urine, feces, and expired air, respectively. The majority of the sofosbuvir dose recovered in urine was GS-331007 (78%) while 3.5% was recovered as sofosbuvir. These data indicate that renal clearance is the major elimination pathway for GS-331007. The median terminal half-lives of sofosbuvir and GS-331007 were 0.4 and 27 hours, respectively.
Specific Populations
Race
Population pharmacokinetics analysis in HCV-infected subjects indicated that race had no clinically relevant effect on the exposure of sofosbuvir and GS-331007.
Gender
No clinically relevant pharmacokinetic differences have been observed between men and women for sofosbuvir and GS-331007.
Pediatric Patients
The pharmacokinetics of sofosbuvir and GS-331007 were determined in HCV genotype 2 or 3 infected pediatric subjects 3 years of age and older receiving a daily dose of SOVALDI as described in Table 8. Exposures in pediatric subjects were similar to those observed in adults.
| Weight Group | Dose | PK Parameter | Geometric Mean (%CV) | ||
|---|---|---|---|---|---|
| Sofosbuvir | GS-331007 | ||||
| ≥35 kg† | 400 mg | AUCtau (ng∙hr/mL) | 1060 (50.6) | 7570 (32.8) | |
| Cmax (ng/mL) | 472 (53.0) | 572 (40.7) | |||
| 17 to <35 kg‡ | 200 mg | AUCtau (ng∙hr/mL) | 891 (36.1) | 10400 (31.6) | |
| Cmax (ng/mL) | 438 (26.4) | 866 (27.1) | |||
| <17 kg§ | 150 mg | AUCtau (ng∙hr/mL) | 851 (41.7) | 9060 (37.6) | |
| Cmax (ng/mL) | 418 (26.8) | 767 (28.3) | |||
| |||||
The pharmacokinetics of sofosbuvir and GS-331007 have not been established in pediatric subjects less than 3 years of age [see Use in Specific Populations (8.4) and Clinical Studies (14.5)].
Geriatric Patients
Population pharmacokinetic analysis in HCV-infected subjects showed that within the age range (19 to 75 years) analyzed, age did not have a clinically relevant effect on the exposure to sofosbuvir and GS-331007 [see Use in Specific Populations (8.5)].
Patients with Renal Impairment
The pharmacokinetics of sofosbuvir were studied in HCV negative subjects with mild (eGFR between 50 to less than 80 mL/min/1.73m2), moderate (eGFR between 30 to less than 50 mL/min/1.73m2), severe renal impairment (eGFR less than 30 mL/min/1.73m2) and subjects with end stage renal disease (ESRD) requiring hemodialysis following a single 400 mg dose of sofosbuvir. Relative to subjects with normal renal function (eGFR greater than 80 mL/min/1.73m2), the sofosbuvir AUC0–inf was 61%, 107% and 171% higher in mild, moderate and severe renal impairment, while the GS-331007 AUC0–inf was 55%, 88% and 451% higher, respectively. In subjects with ESRD, relative to subjects with normal renal function, sofosbuvir and GS-331007 AUC0–inf was 28% and 1280% higher when sofosbuvir was dosed 1 hour before hemodialysis compared with 60% and 2070% higher when sofosbuvir was dosed 1 hour after hemodialysis, respectively. A 4 hour hemodialysis session removed approximately 18% of administered dose. No dosage adjustment is required for patients with mild or moderate renal impairment. The safety and efficacy of SOVALDI have not been established in patients with severe renal impairment or ESRD. No dosage recommendation can be given for patients with severe renal impairment or ESRD [see Dosage and Administration (2.6) and Use in Specific Populations (8.6)].
Patients with Hepatic Impairment
The pharmacokinetics of sofosbuvir were studied following 7-day dosing of 400 mg sofosbuvir in HCV-infected subjects with moderate and severe hepatic impairment (Child-Pugh Class B and C). Relative to subjects with normal hepatic function, the sofosbuvir AUC0–24 were 126% and 143% higher in moderate and severe hepatic impairment, while the GS-331007 AUC0–24 were 18% and 9% higher, respectively. Population pharmacokinetics analysis in HCV-infected subjects indicated that cirrhosis had no clinically relevant effect on the exposure of sofosbuvir and GS-331007. No dosage adjustment of SOVALDI is recommended for patients with mild, moderate or severe hepatic impairment [see Use in Specific Populations (8.7)].
Assessment of Drug Interactions
Sofosbuvir is a substrate of drug transporter P-gp and breast cancer resistance protein (BCRP) while GS-331007 is not. Drugs that are P-gp inducers in the intestine (e.g., rifampin or St. John's wort) may decrease sofosbuvir plasma concentration, leading to reduced therapeutic effect of SOVALDI, and thus concomitant use with SOVALDI is not recommended [see Warnings and Precautions (5.3) and Drug Interactions (7.1)].
Coadministration of SOVALDI with drugs that inhibit P-gp and/or BCRP may increase sofosbuvir plasma concentration without increasing GS-331007 plasma concentration; accordingly, SOVALDI may be coadministered with P-gp and/or BCRP inhibitors. Sofosbuvir and GS-331007 are not inhibitors of P-gp and BCRP and thus are not expected to increase exposures of drugs that are substrates of these transporters.
The intracellular metabolic activation pathway of sofosbuvir is mediated by generally low affinity and high capacity hydrolase and nucleotide phosphorylation pathways that are unlikely to be affected by concomitant drugs.
The effects of coadministered drugs on the exposure of sofosbuvir and GS-331007 are shown in Table 9. The effects of sofosbuvir on the exposure of coadministered drugs are shown in Table 10 [see Drug Interactions (7.1, 7.2)].
| Coadministered Drug | Dose of Coadministered Drug (mg) | Sofosbuvir Dose (mg) | N | Mean Ratio (90% CI) of Sofosbuvir and GS-331007 PK With/Without Coadministered Drug No Effect=1.00 | ||||
|---|---|---|---|---|---|---|---|---|
| Cmax | AUC | Cmin | ||||||
| NA = not available/not applicable | ||||||||
| ||||||||
| Cyclosporine | 600 single dose | 400 single dose | 19 | sofosbuvir | 2.54 (1.87, 3.45) | 4.53 (3.26, 6.30) | NA | |
| GS-331007 | 0.60 (0.53, 0.69) | 1.04 (0.90, 1.20) | NA | |||||
| Darunavir (boosted with ritonavir) | 800/100 once daily | 400 single dose | 18 | sofosbuvir | 1.45 (1.10, 1.92) | 1.34 (1.12, 1.59) | NA | |
| GS-331007 | 0.97 (0.90, 1.05) | 1.24 (1.18, 1.30) | NA | |||||
| Efavirenz† | 600 once daily | 400 single dose | 16 | sofosbuvir | 0.81 (0.60, 1.10) | 0.94 (0.76, 1.16) | NA | |
| Emtricitabine† | 200 once daily | |||||||
| Tenofovir disoproxil fumarate† | 300 once daily | GS-331007 | 0.77 (0.70, 0.84) | 0.84 (0.76, 0.92) | NA | |||
| Methadone | 30 to 130 once daily | 400 once daily | 14 | sofosbuvir | 0.95‡
(0.68, 1.33) | 1.30‡
(1.00, 1.69) | NA | |
| GS-331007 | 0.73‡
(0.65, 0.83) | 1.04‡
(0.89, 1.22) | NA | |||||
| Rilpivirine | 25 once daily | 400 single dose | 17 | sofosbuvir | 1.21 (0.90, 1.62) | 1.09 (0.94, 1.27) | NA | |
| GS-331007 | 1.06 (0.99, 1.14) | 1.01 (0.97, 1.04) | NA | |||||
| Tacrolimus | 5 single dose | 400 single dose | 16 | sofosbuvir | 0.97 (0.65, 1.43) | 1.13 (0.81, 1.57) | NA | |
| GS-331007 | 0.97 (0.83, 1.14) | 1.00 (0.87, 1.13) | NA | |||||
No effect on the pharmacokinetic parameters of sofosbuvir and GS-331007 was observed with raltegravir.
| Coadministered Drug | Dose of Coadministered Drug (mg) | Sofosbuvir Dose (mg) | N | Mean Ratio (90% CI) of Coadministered Drug PK With/Without Sofosbuvir No Effect=1.00 | |||
|---|---|---|---|---|---|---|---|
| Cmax | AUC | Cmin | |||||
| NA = not available/not applicable | |||||||
| |||||||
| Norelgestromin | norgestimate 0.18/0.215/0.25/ ethinyl estradiol 0.025 once daily | 400 once daily | 15 | 1.07 (0.94, 1.22) | 1.06 (0.92, 1.21) | 1.07 (0.89, 1.28) | |
| Norgestrel | 1.18 (0.99, 1.41) | 1.19 (0.98, 1.45) | 1.23 (1.00, 1.51) | ||||
| Ethinyl estradiol | 1.15 (0.97, 1.36) | 1.09 (0.94, 1.26) | 0.99 (0.80, 1.23) | ||||
| Raltegravir | 400 twice daily | 400 single dose | 19 | 0.57 (0.44, 0.75) | 0.73 (0.59, 0.91) | 0.95 (0.81, 1.12) | |
| Tacrolimus | 5 single dose | 400 single dose | 16 | 0.73 (0.59, 0.90) | 1.09 (0.84, 1.40) | NA | |
| Tenofovir disoproxil fumarate† | 300 once daily | 400 single dose | 16 | 1.25 (1.08, 1.45) | 0.98 (0.91, 1.05) | 0.99 (0.91, 1.07) | |
No effect on the pharmacokinetic parameters of the following coadministered drugs was observed with sofosbuvir: cyclosporine, darunavir/ritonavir, efavirenz, emtricitabine, methadone, or rilpivirine.
12.4 Microbiology
Mechanism of Action
Sofosbuvir is an inhibitor of the HCV NS5B RNA-dependent RNA polymerase, which is essential for viral replication. Sofosbuvir is a nucleotide prodrug that undergoes intracellular metabolism to form the pharmacologically active uridine analog triphosphate (GS-461203), which can be incorporated into HCV RNA by the NS5B polymerase and acts as a chain terminator. In a biochemical assay, GS-461203 inhibited the polymerase activity of the recombinant NS5B from HCV genotype 1b, 2a, 3a and 4a with IC50 values ranging from 0.7 to 2.6 micromolar. GS-461203 is neither an inhibitor of human DNA and RNA polymerases nor an inhibitor of mitochondrial RNA polymerase.
Antiviral Activity
In HCV replicon assays, the EC50 values of sofosbuvir against full-length replicons from genotype 1a, 1b, 2a, 3a and 4a, and chimeric 1b replicons encoding NS5B from genotype 2b, 5a or 6a ranged from 0.014 to 0.11 micromolar. The median EC50 value of sofosbuvir against chimeric replicons encoding NS5B sequences from clinical isolates was 0.062 micromolar for genotype 1a (range 0.029–0.128 micromolar; N=67), 0.102 micromolar for genotype 1b (range 0.045–0.170 micromolar; N=29), 0.029 micromolar for genotype 2 (range 0.014–0.081 micromolar; N=15) and 0.081 micromolar for genotype 3a (range 0.024–0.181 micromolar; N=106). In infectious virus assays, the EC50 values of sofosbuvir against genotype 1a and 2a were 0.03 and 0.02 micromolar, respectively. The presence of 40% human serum had no effect on the anti-HCV activity of sofosbuvir. Evaluation of sofosbuvir in combination with interferon alpha or ribavirin showed no antagonistic effect in reducing HCV RNA levels in replicon cells.
Resistance
In Cell Culture
HCV replicons with reduced susceptibility to sofosbuvir have been selected in cell culture for multiple genotypes including 1b, 2a, 2b, 3a, 4a, 5a and 6a. Reduced susceptibility to sofosbuvir was associated with the primary NS5B substitution S282T in all replicon genotypes examined. An M289L substitution developed along with the S282T substitution in genotype 2a, 5 and 6 replicons. Site-directed mutagenesis of the S282T substitution in replicons of 8 genotypes conferred 2- to 18-fold reduced susceptibility to sofosbuvir and reduced the replication viral capacity by 89% to 99% compared to the corresponding wild-type. In biochemical assays, recombinant NS5B polymerase from genotypes 1b, 2a, 3a and 4a expressing the S282T substitution showed reduced susceptibility to GS-461203 compared to respective wild-types.
In Clinical Trials
In a pooled analysis of 982 subjects who received SOVALDI in Phase 3 trials, 224 subjects had post-baseline NS5B genotypic data from next generation nucleotide sequencing (assay cutoff of 1%).
Treatment-emergent substitutions L159F (n=6) and V321A (n=5) were detected in post-baseline samples from GT3a-infected subjects across the Phase 3 trials. No detectable shift in the phenotypic susceptibility to sofosbuvir of subject isolates with L159F or V321A substitutions was seen. The sofosbuvir-associated resistance substitution S282T was not detected at baseline or in the failure isolates from Phase 3 trials. However, an S282T substitution was detected in one genotype 2b subject who relapsed at Week 4 post-treatment after 12 weeks of sofosbuvir monotherapy in the Phase 2 trial P7977-0523 [ELECTRON]. The isolate from this subject displayed a mean 13.5-fold reduced susceptibility to sofosbuvir. For this subject, the S282T substitution was no longer detectable at Week 12 post-treatment by next generation sequencing with an assay cutoff of 1%.
In the trial done in subjects with hepatocellular carcinoma awaiting liver transplantation where subjects received up to 48 weeks of sofosbuvir and ribavirin, the L159F substitution emerged in multiple subjects with GT1a or GT2b HCV who experienced virologic failure (breakthrough and relapse). Furthermore, the presence of substitutions L159F and/or C316N at baseline was associated with sofosbuvir breakthrough and relapse post-transplant in multiple subjects infected with GT1b HCV. In addition, S282R and L320F substitutions were detected on-treatment by next generation sequencing in a subject infected with GT1a HCV with a partial treatment response.
The clinical significance of these substitutions is not known.
Cross Resistance
HCV replicons expressing the sofosbuvir-associated resistance substitution S282T were susceptible to NS5A inhibitors and ribavirin. HCV replicons expressing the ribavirin-associated substitutions T390I and F415Y were susceptible to sofosbuvir. Sofosbuvir was active against HCV replicons with NS3/4A protease inhibitor, NS5B non-nucleoside inhibitor and NS5A inhibitor resistant variants.
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis and Mutagenesis
Use with Ribavirin and/or Peginterferon alfa: Refer to prescribing information for ribavirin and/or peginterferon alfa for information on carcinogenesis and mutagenesis.
Sofosbuvir was not genotoxic in a battery of in vitro or in vivo assays, including bacterial mutagenicity, chromosome aberration using human peripheral blood lymphocytes and in vivo mouse micronucleus assays.
Two-year carcinogenicity studies in mice and rats were conducted with sofosbuvir. Mice were administered doses of up to 200 mg/kg/day in males and 600 mg/kg/day in females, while rats were administered doses of up to 750 mg/kg/day in males and females. No increase in the incidence of drug-related neoplasms were observed at the highest doses tested in mice and rats, resulting in AUC exposure to the predominant circulating metabolite GS-331007 of approximately 7 and 30 times (in mice) and 13 and 17 times (in rats), in males and females respectively, the exposure in humans at the recommended clinical dose.
Impairment of Fertility
Use with Ribavirin and/or Peginterferon alfa: Refer to prescribing information for ribavirin and/or peginterferon alfa for information on impairment of fertility.
Sofosbuvir had no effects on embryo-fetal viability or on fertility when evaluated in rats. At the highest dose tested, AUC exposure to the predominant circulating metabolite GS-331007 was approximately 8 times the exposure in humans at the recommended clinical dose.
14. Clinical Studies
14.1 Description of Clinical Trials
The safety and efficacy of SOVALDI was evaluated in five Phase 3 trials in a total of 1724 HCV mono-infected subjects with genotypes 1 to 6 chronic hepatitis C virus, one Phase 3 trial in 223 HCV/HIV-1 coinfected subjects with genotype 1, 2 or 3 HCV, and one trial in 106 pediatric subjects 3 years of age and older with genotype 2 or 3 HCV, as summarized in Table 11 [see Clinical Studies (14.2, 14.3, 14.4, and 14.5)].
| Trial | Population | Study Arms (Number of Subjects Treated) |
|---|---|---|
| NEUTRINO *
(NCT01641640) | Treatment naïve (TN) (GT1, 4, 5 or 6) | SOVALDI+Peg-IFN alfa+RBV 12 weeks (327) |
| FISSION *
(NCT01497366) | TN (GT2 or 3) | SOVALDI+RBV 12 Weeks (256) Peg-IFN alfa+RBV 24 weeks (243) |
| POSITRON †
(NCT01542788) | Interferon intolerant, ineligible or unwilling subjects (GT2 or 3) | SOVALDI+RBV 12 Weeks (207) Placebo 12 weeks (71) |
| FUSION †
(NCT01604850) | Previous interferon relapsers or nonresponders (GT2 or 3) | SOVALDI+RBV 12 Weeks (103) SOVALDI+RBV 16 Weeks (98) |
| VALENCE †
(NCT01682720) | TN or previous interferon relapsers or nonresponders (GT2 or 3) | SOVALDI+RBV 12 Weeks for GT2 (73) SOVALDI+RBV 12 Weeks for GT3 (11) SOVALDI+RBV 24 Weeks for GT3 (250) Placebo for 12 weeks (85) |
| PHOTON-1 *
(NCT01667731) |
| SOVALDI+RBV 24 Weeks for GT1 (114) SOVALDI+RBV 12 Weeks for GT2 or 3 TN (68) SOVALDI+RBV 24 Weeks for GT2 or 3 previous interferon relapsers or nonresponders (41) |
| 1112 (NCT02175758)* | GT2 or GT3 pediatric subjects 3 years of age and older | SOVALDI+RBV 12 Weeks for GT2 (31) SOVALDI+RBV 24 Weeks for GT3 (75) |
| ||
Subjects in the adult trials did not have cirrhosis or had compensated cirrhosis. SOVALDI was administered at a dose of 400 mg once daily. The ribavirin (RBV) dosage for adult subjects was weight-based at 1000–1200 mg daily administered in two divided doses when used in combination with SOVALDI, and the peginterferon alfa 2a dosage, where applicable, was 180 micrograms per week. Treatment duration was fixed in each trial and was not guided by subjects' HCV RNA levels (no response guided algorithm). Plasma HCV RNA values were measured during the clinical trials using the COBAS TaqMan HCV test (version 2.0), for use with the High Pure System. The assay had a lower limit of quantification (LLOQ) of 25 IU per mL. Sustained virologic response (SVR12) was the primary endpoint which was defined as HCV RNA less than LLOQ at 12 weeks after the end of treatment.
14.2 Clinical Trials in Subjects with Genotype 1 or 4 HCV
Treatment-Naïve Adults ─ NEUTRINO (Study 110)
NEUTRINO was an open-label, single-arm trial that evaluated 12 weeks of treatment with SOVALDI in combination with peginterferon alfa 2a and ribavirin in treatment-naïve subjects with genotype 1, 4, 5 or 6 HCV infection compared to pre-specified historical control.
Treated subjects (N=327) had a median age of 54 years (range: 19 to 70); 64% of the subjects were male; 79% were White, 17% were Black; 14% were Hispanic or Latino; mean body mass index was 29 kg/m2 (range: 18 to 56 kg/m2); 78% had baseline HCV RNA greater than 6 log10 IU per mL; 17% had cirrhosis; 89% had HCV genotype 1; 9% had HCV genotype 4 and 2% had HCV genotype 5 or 6. Table 12 presents the SVR12 for the treatment group of SOVALDI + peginterferon alfa + ribavirin in subjects with genotype 1 or 4 HCV. Available data on subjects with genotype 5 or 6 HCV treated with SOVALDI + peginterferon alfa + ribavirin for 12 weeks were insufficient for dosing recommendations; therefore these results are not presented in Table 12 [see Use in Specific Populations (8.10)].
| SOVALDI + Peg-IFN alfa + RBV 12 weeks | |||
|---|---|---|---|
| N=320 | |||
| Overall SVR | 90% (289/320) | ||
| Genotype 1* | 90% (262/292) | ||
| Genotype 1a | 92% (206/225) | ||
| Genotype 1b | 83% (55/66) | ||
| Genotype 4 | 96% (27/28) | ||
| Outcome for subjects without SVR | |||
| On-treatment virologic failure | 0/320 | ||
| Relapse† | 9% (28/319) | ||
| Other‡ | 1% (3/320) | ||
| |||
SVR12 for selected subgroups are presented in Table 13.
| SOVALDI + Peg-IFN alfa + RBV 12 weeks | |
|---|---|
| Cirrhosis | |
| No | 93% (247/267) |
| Yes | 79% (42/53) |
| Race | |
| Black | 87% (47/54) |
| Non-black | 91% (242/266) |
| Multiple Baseline Factors | |
| Genotype 1, Metavir F3/F4 fibrosis, IL28B non-C/C, HCV RNA >800,000 IU/mL | 71% (37/52) |
SVR12 rates were 99% (89/90) in subjects with genotype 1 or 4 HCV and baseline IL28B C/C allele and 87% (200/230) in subjects with genotype 1 or 4 HCV and baseline IL28B non-C/C alleles.
It is estimated that the SVR12 in patients who previously failed pegylated interferon and ribavirin therapy will approximate the observed SVR12 in NEUTRINO subjects with multiple baseline factors traditionally associated with a lower response to interferon-based treatment (Table 13).
The SVR12 rate in the NEUTRINO trial in genotype 1 subjects with IL28B non-C/C alleles, HCV RNA greater than 800,000 IU/mL and Metavir F3/F4 fibrosis was 71% (37/52).
14.3 Clinical Trials in Subjects with Genotype 2 or 3 HCV
Treatment-Naïve Adults ─ FISSION (Study 1231)
FISSION was a randomized, open-label, active-controlled trial that evaluated 12 weeks of treatment with SOVALDI and ribavirin compared to 24 weeks of treatment with peginterferon alfa 2a and ribavirin in treatment-naïve subjects with genotype 2 and 3 HCV. The ribavirin dosage used in the SOVALDI + ribavirin and peginterferon alfa 2a + ribavirin arms were weight-based 1000–1200 mg per day and 800 mg per day regardless of weight, respectively. Subjects were randomized in a 1:1 ratio and stratified by cirrhosis (presence vs. absence), HCV genotype (2 vs. 3) and baseline HCV RNA level (less than 6 log10 IU/mL vs. at least 6 log10 IU/mL). Subjects with genotype 2 or 3 HCV were enrolled in an approximately 1:3 ratio.
Treated subjects (N=499) had a median age of 50 years (range: 19 to 77); 66% of the subjects were male; 87% were White, 3% were Black; 14% were Hispanic or Latino; mean body mass index was 28 kg/m2 (range: 17 to 52 kg/m2); 57% had baseline HCV RNA levels greater than 6 log10 IU per mL; 20% had cirrhosis; 72% had HCV genotype 3. Table 14 presents the SVR12 for the treatment groups of SOVALDI + ribavirin and peginterferon alfa + ribavirin in subjects with genotype 2 HCV. SVR12 for genotype 3 subjects treated with SOVALDI + ribavirin for 12 weeks was suboptimal; therefore these results are not presented in Table 14.
| SOVALDI + RBV 12 weeks | Peg-IFN alfa + RBV 24 weeks | |||
|---|---|---|---|---|
| N=73* | N=67* | |||
| SVR12 | 95% (69/73) | 78% (52/67) | ||
| Outcome for subjects without SVR12 | ||||
| On-treatment virologic failure | 0/73 | 4% (3/67) | ||
| Relapse† | 5% (4/73) | 15% (9/62) | ||
| Other‡ | 0/73 | 4% (3/67) | ||
| ||||
SVR12 for genotype 2 HCV-infected subjects with cirrhosis at baseline are presented in Table 15.
| SOVALDI + RBV 12 weeks | Peg-IFN alfa + RBV 24 weeks | |
|---|---|---|
| N=73 | N=67 | |
| Cirrhosis | ||
| No | 97% (59/61) | 81% (44/54) |
| Yes | 83% (10/12) | 62% (8/13) |
Interferon Intolerant, Ineligible or Unwilling Adults ─ POSITRON (Study 0107)
POSITRON was a randomized, double-blinded, placebo-controlled trial that evaluated 12 weeks of treatment with SOVALDI and ribavirin (N=207) compared to placebo (N=71) in subjects who are interferon intolerant, ineligible or unwilling. Subjects were randomized in 3:1 ratio and stratified by cirrhosis (presence vs. absence).
Treated subjects (N=278) had a median age of 54 years (range: 21 to 75); 54% of the subjects were male; 91% were White, 5% were Black; 11% were Hispanic or Latino; mean body mass index was 28 kg/m2 (range: 18 to 53 kg/m2); 70% had baseline HCV RNA levels greater than 6 log10 IU per mL; 16% had cirrhosis; 49% had HCV genotype 3. The proportions of subjects who were interferon intolerant, ineligible, or unwilling were 9%, 44%, and 47%, respectively. Most subjects had no prior HCV treatment (81%). Table 16 presents the SVR12 for the treatment groups of SOVALDI + ribavirin and placebo in subjects with genotype 2 HCV. SVR12 for genotype 3 subjects treated with SOVALDI + ribavirin for 12 weeks was suboptimal; therefore these results are not presented in Table 16.
| SOVALDI + RBV 12 weeks | Placebo 12 weeks | |||
|---|---|---|---|---|
| N=109 | N= 34 | |||
| SVR12 | 93% (101/109) | 0/34 | ||
| Outcome for subjects without SVR12 | ||||
| On-treatment virologic failure | 0/109 | 97% (33/34) | ||
| Relapse* | 5% (5/107) | 0/0 | ||
| Other† | 3% (3/109) | 3% (1/34) | ||
| ||||
Table 17 presents the subgroup analysis for cirrhosis and interferon classification in subjects with genotype 2 HCV.
| SOVALDI + RBV 12 weeks | |
|---|---|
| N=109 | |
| Cirrhosis | |
| No | 92% (85/92) |
| Yes | 94% (16/17) |
| Interferon Classification | |
| Ineligible | 88% (36/41) |
| Intolerant | 100% (9/9) |
| Unwilling | 95% (56/59) |
Previously Treated Adults ─ FUSION (Study 0108)
FUSION was a randomized, double-blinded trial that evaluated 12 or 16 weeks of treatment with SOVALDI and ribavirin in subjects who did not achieve SVR with prior interferon-based treatment (relapsers and nonresponders). Subjects were randomized in a 1:1 ratio and stratified by cirrhosis (presence vs. absence) and HCV genotype (2 vs. 3).
Treated subjects (N=201) had a median age of 56 years (range: 24 to 70); 70% of the subjects were male; 87% were White; 3% were Black; 9% were Hispanic or Latino; mean body mass index was 29 kg/m2 (range: 19 to 44 kg/m2); 73% had baseline HCV RNA levels greater than 6 log10 IU per mL; 34% had cirrhosis; 63% had HCV genotype 3; 75% were prior relapsers. Table 18 presents the SVR12 for the treatment groups of SOVALDI + ribavirin for 12 weeks in subjects with genotype 2 HCV. Treatment of 16 weeks in subjects with genotype 2 HCV was not shown to increase the SVR12 observed with 12 weeks of treatment. SVR12 for genotype 3 subjects treated with SOVALDI + ribavirin for 12 or 16 weeks was suboptimal; therefore these results are not presented in Table 18.
| SOVALDI + RBV 12 weeks | |||
|---|---|---|---|
| N=39* | |||
| SVR12 | 82% (32/39) | ||
| Outcome for subjects without SVR12 | |||
| On-treatment virologic failure | 0/39 | ||
| Relapse† | 18% (7/39) | ||
| Other‡ | 0/39 | ||
| |||
Table 19 presents the subgroup analysis for cirrhosis and response to prior HCV treatment in subjects with genotype 2 HCV.
| SOVALDI + RBV 12 weeks | |
|---|---|
| N=39 | |
| Cirrhosis | |
| No | 90% (26/29) |
| Yes | 60% (6/10) |
| Response to prior HCV treatment | |
| Relapser/ breakthrough | 86% (25/29) |
| Nonresponder | 70% (7/10) |
Treatment-Naïve and Previously Treated Adults ─ VALENCE (Study 0133)
The VALENCE trial evaluated SOVALDI in combination with weight-based ribavirin for the treatment of genotype 2 or 3 HCV infection in treatment-naïve subjects or subjects who did not achieve SVR with prior interferon-based treatment, including subjects with compensated cirrhosis. The original trial design was a 4 to 1 randomization to SOVALDI + ribavirin for 12 weeks or placebo. Based on emerging data, this trial was unblinded and all genotype 2 HCV-infected subjects continued the original planned treatment and received SOVALDI + ribavirin for 12 weeks, and duration of treatment with SOVALDI + ribavirin in genotype 3 HCV-infected subjects was extended to 24 weeks. Eleven genotype 3 subjects had already completed SOVALDI + ribavirin for 12 weeks at the time of the amendment.
Treated subjects (N=419) had a median age of 51 years (range: 19 to 74); 60% of the subjects were male; mean body mass index was 26 kg/m2 (range: 17 to 44 kg/m2); the mean baseline HCV RNA level was 6.4 log10 IU per mL; 78% had HCV genotype 3; 58% of the subjects were treatment-experienced and 65% of those subjects experienced relapse/breakthrough to prior HCV treatment.
Table 20 presents the SVR12 for the treatment groups of SOVALDI + ribavirin for 12 weeks and 24 weeks.
| Genotype 2 SOVALDI + RBV 12 weeks | Genotype 3 SOVALDI + RBV 24 weeks | ||||
|---|---|---|---|---|---|
| N=73 | N=250 | ||||
| Overall SVR | 93% (68/73) | 84% (210/250) | |||
| Outcome for subjects without SVR | |||||
| On-treatment virologic failure | 0% (0/73) | <1% (1/250) | |||
| Relapse† | 7% (5/73) | 14% (34/249) | |||
| Treatment-naïve | 3% (1/32) | 5% (5/105) | |||
| Treatment-experienced | 10% (4/41) | 20% (29/144) | |||
| Other‡ | 0% (0/73) | 2% (5/250) | |||
| |||||
Table 21 presents the subgroup analysis by genotype for cirrhosis and prior HCV treatment experience.
| Genotype 2 SOVALDI + RBV 12 weeks | Genotype 3 SOVALDI + RBV 24 weeks | |
|---|---|---|
| N=73 | N=250 | |
| Treatment-naïve | 97% (31/32) | 93% (98/105) |
| Non-cirrhotic | 97% (29/30) | 93% (86/92) |
| Cirrhotic | 100% (2/2) | 92% (12/13) |
| Treatment-experienced | 90% (37/41) | 77% (112/145) |
| Non-cirrhotic | 91% (30/33) | 85% (85/100) |
| Cirrhotic | 88% (7/8) | 60% (27/45) |
14.4 Clinical Trials in Adult Subjects Coinfected with HCV and HIV-1 ─ Photon-1 (Study 0123)
SOVALDI was studied in an open-label clinical trial (Study PHOTON-1) evaluating the safety and efficacy of 12 or 24 weeks of treatment with SOVALDI and ribavirin in adult subjects with genotype 1, 2 or 3 chronic hepatitis C coinfected with HIV-1. Genotype 2 and 3 subjects were either HCV treatment-naïve or experienced, whereas genotype 1 subjects were all treatment-naïve. Subjects received 400 mg SOVALDI and weight-based ribavirin (1000 mg for subjects weighing less than 75 kg or 1200 mg for subjects weighing at least 75 kg) daily for 12 or 24 weeks based on genotype and prior treatment history. Subjects were either not on antiretroviral therapy with a CD4+ cell count greater than 500 cells/mm3 or had virologically suppressed HIV-1 with a CD4+ cell count greater than 200 cells/mm3. Efficacy data 12 weeks post treatment are available for 210 subjects (see Table 22).
| HCV genotype 1 | HCV genotype 2 | HCV genotype 3 | |||||
|---|---|---|---|---|---|---|---|
| SOVALDI + RBV 24 weeks TN (N=114) | SOVALDI + RBV 12 weeks TN (N=26) | SOVALDI + RBV 24 weeks TE (N=13) | |||||
| TN = Treatment-naïve; TE = Treatment-experienced | |||||||
| |||||||
| Overall | 76% (87/114) | 88% (23/26) | 92% (12/13) | ||||
| Outcome for subjects without SVR12 | |||||||
| On-treatment virologic failure | 1% (1/114) | 4% (1/26) | 0/13 | ||||
| Relapse† | 22% (25/113) | 0/25 | 8% (1/13) | ||||
| Other‡ | 1% (1/114) | 8% (2/26) | 0/13 | ||||
In subjects with HCV genotype 1 infection, the SVR12 rate was 82% (74/90) in subjects with genotype 1a infection and 54% (13/24) in subjects with genotype 1b infection, with relapse accounting for the majority of treatment failures. SVR12 rates in subjects with HCV genotype 1 infection were 80% (24/30) in subjects with baseline IL28B C/C allele and 75% (62/83) in subjects with baseline IL28B non-C/C alleles.
In the 223 HCV subjects with HIV-1 coinfection, the percentage of CD4+ cells did not change during treatment. Median CD4+ cell count decreases of 85 cells/mm3 and 84 cells/mm3 were observed at the end of treatment with SOVALDI + ribavirin for 12 or 24 weeks, respectively. HIV-1 rebound during SOVALDI + ribavirin treatment occurred in 2 subjects (0.9%) on antiretroviral therapy.
14.5 Clinical Trial in Pediatrics (Study 1112)
The efficacy of SOVALDI in HCV-infected pediatric subjects 3 years of age and older was evaluated in 106 subjects with HCV genotype 2 (N = 31) or genotype 3 (N = 75) in a Phase 2, open label clinical trial. Subjects with HCV genotype 2 or 3 infection in the trial were treated with SOVALDI and weight-based ribavirin for 12 or 24 weeks, respectively [see Dosage and Administration (2.3)].
Subjects 12 Years to <18 Years of Age: SOVALDI was evaluated in 52 subjects12 years to <18 years of age with HCV genotype 2 (N = 13) or genotype 3 (N = 39) infection. The median age was 15 years (range: 12 to 17); 40% of the subjects were female; 90% were White, 4% were Black, and 2% were Asian; 4% were Hispanic/Latino; mean body mass index was 22 kg/m2 (range: 16 to 32 kg/m2);mean weight was 60 kg (range: 30 to 101 kg); 17% were treatment experienced; 65% had baseline HCV RNA levels greater than or equal to 800,000 IU/mL; and no subjects had known cirrhosis. The majority of subjects (71%) had been infected through vertical transmission.
The SVR12 rate was 100% [13/13] in genotype 2 subjects and 97% [38/39] in genotype 3 subjects. No subject experienced on-treatment virologic failure or relapse.
Subjects 6 Years to <12 Years of Age: SOVALDI was evaluated in 41 subjects 6 years to <12 years of age with HCV genotype 2 (N = 13) or genotype 3 (N = 28) infection. The median age was 9 years (range: 6 to 11); 73% of the subjects were female; 71% were White and 20% were Asian; 15% were Hispanic/Latino; mean body mass index was 19 kg/m2 (range: 13 to 32 kg/m2); mean weight was 34 kg (range 15 to 80 kg); 98% were treatment naive; 46% had baseline HCV RNA levels greater than or equal to 800,000 IU/mL; and no subjects had known cirrhosis. The majority of subjects (98%) had been infected through vertical transmission.
The SVR12 rate was 100% (13/13) in genotype 2 and 100% (28/28) in genotype 3 subjects.). No subjects experienced on-treatment virologic failure or relapse.
Subjects 3 Years to <6 Years of Age: SOVALDI was evaluated in 13 subjects 3 years to <6 years of age with HCV genotype 2 (N = 5) or genotype 3 (N = 8) infection. The median age was 4 years (range: 3 to 5); 77% of the subjects were female; 69% were White, 8% were Black, and 8% were Asian; 8% were Hispanic/Latino; mean body mass index was 15 kg/m2 (range: 13 to 17 kg/m2); mean weight was 17 kg (range 13 to 19 kg); 100% were treatment naive; 23% had baseline HCV RNA levels greater than or equal to 800,000 IU/mL; and no subjects had known cirrhosis. The majority of subjects (85%) had been infected through vertical transmission.
The SVR12 rate was 80% (4/5) in genotype 2 subjects and 100% (8/8) in genotype 3 subjects. No subjects experienced on-treatment virologic failure or relapse. One subject prematurely discontinued study treatment due to an adverse event.
16. How Supplied/Storage and Handling
Tablets
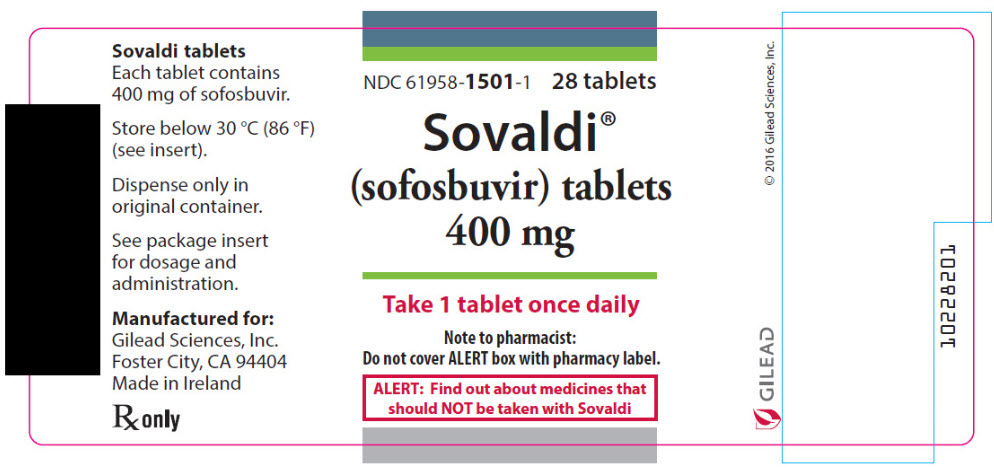
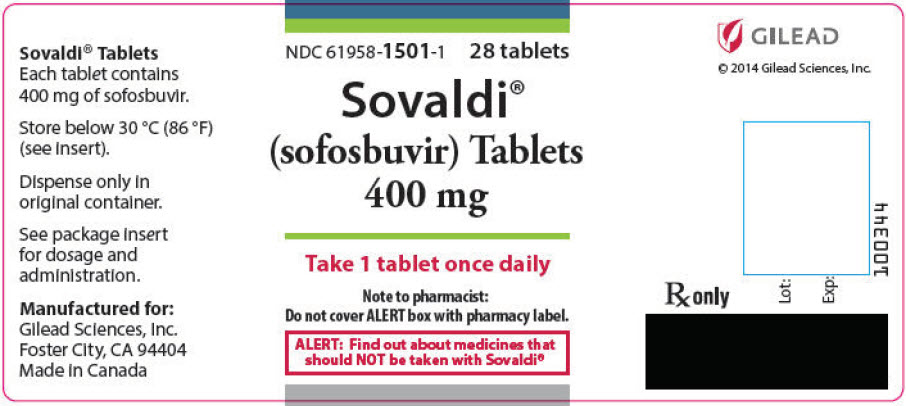
SOVALDI tablets, 400 mg, are yellow, capsule-shaped, film-coated tablets containing 400 mg sofosbuvir debossed with "GSI" on one side and "7977" on the other side. Each bottle contains 28 tablets (NDC 61958-1501-1), a silica gel desiccant and polyester coil with a child-resistant closure.
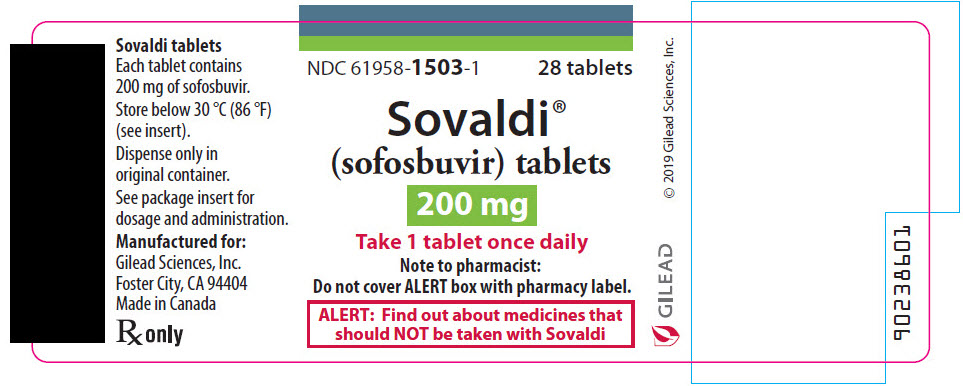
SOVALDI tablets, 200 mg, are yellow, oval-shaped, film-coated tablets containing 200 mg sofosbuvir debossed with "GSI" on one side and "200" on the other side. Each bottle contains 28 tablets (NDC 61958-1503-1) and a polyester coil with a child-resistant closure.
Store below 30 °C (86 °F).
- Dispense only in original container
- Do not use if seal over bottle opening is broken or missing
Oral Pellets
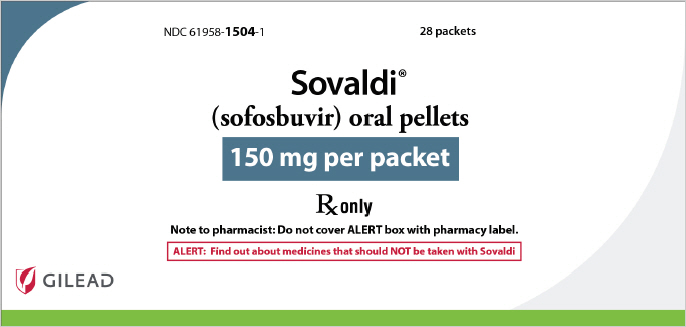
SOVALDI pellets, 150 mg, are white to off-white pellets supplied as unit-dose packets in cartons. Each carton contains 28 packets (NDC 61958-1504-1)
SOVALDI pellets, 200 mg, are white to off-white pellets supplied as unit-dose packets in cartons. Each carton contains 28 packets (NDC 61958-1505-1)
- Store below 30 °C (86 °F).
- Do not use if carton tamper-evident or packet seal is broken or damaged.
17. Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
Risk of Hepatitis B Virus Reactivation in Patients Coinfected with HCV and HBV
Inform patients that HBV reactivation can occur in patients coinfected with HBV during or after treatment of HCV infection. Advise patients to tell their healthcare provider if they have a history of HBV infection [see Warnings and Precautions (5.1)].
Serious Symptomatic Bradycardia When Coadministered with Amiodarone
Advise patients to seek medical evaluation immediately for symptoms of bradycardia such as near-fainting or fainting, dizziness or lightheadedness, malaise, weakness, excessive tiredness, shortness of breath, chest pain, confusion or memory problems [see Warnings and Precautions (5.2), Adverse Reactions (6.2), and Drug Interactions (7.1)].
Pregnancy
Advise patients to avoid pregnancy during combination treatment with SOVALDI and ribavirin or SOVALDI and peginterferon and ribavirin. Inform patients to notify their health care provider immediately in the event of a pregnancy [see Use in Specific Populations (8.1)].
Drug Interactions
Advise patients that SOVALDI may interact with some drugs; therefore, patients should be advised to report the use of any prescription, non-prescription medication or herbal products to their healthcare provider [see Warnings and Precautions (5.3) and Drug Interactions (7.1)].
Hepatitis C Virus Transmission
Inform patients that the effect of treatment of hepatitis C infection on transmission is not known, and that appropriate precautions to prevent transmission of the hepatitis C virus during treatment or in the event of treatment failure should be taken.
Administration
Advise patients to take SOVALDI every day at the regularly scheduled time with or without food. Inform patients that it is important not to miss or skip doses and to take SOVALDI for the duration that is recommended by the physician.
For SOVALDI oral pellets, advise patients or caregivers to read and follow the Instructions for Use for preparing the correct dose.
Important Information on Coadministration with Ribavirin or Peginterferon and Ribavirin
Advise patients that the recommended regimen for patients with genotype 1 or 4 HCV infection is SOVALDI administered in combination with peginterferon alfa and ribavirin and the recommended regimen for patients with genotype 2 or 3 HCV infection is SOVALDI administered in combination with ribavirin. If peginterferon and/or ribavirin are permanently discontinued, SOVALDI should also be discontinued.
Spl Unclassified Section
Manufactured and distributed by:Gilead Sciences, Inc.Foster City, CA 94404
SOVALDI and HARVONI are trademarks of Gilead Sciences, Inc., or its related companies. All other trademarks referenced herein are the property of their respective owners.
©2024 Gilead Sciences, Inc. All rights reserved.
204671-GS-0011
Patient Package Insert
| This Patient Information has been approved by the U.S. Food and Drug Administration. | Revised: 03/2020 | |||
| Patient Information | ||||
| SOVALDI® (soh-VAHL-dee) (sofosbuvir) tablets | SOVALDI® (soh-VAHL-dee) (sofosbuvir) oral pellets | |||
| Important: SOVALDI is used in combination with other antiviral medicines. When taking SOVALDI with ribavirin or in combination with peginterferon alfa and ribavirin you should also read those Medication Guides. The information in this Patient Information Leaflet talks about SOVALDI when it is used with ribavirin and in combination with peginterferon alfa and ribavirin. | ||||
What is the most important information I should know about SOVALDI?SOVALDI can cause serious side effects, including:
| ||||
| What is SOVALDI? SOVALDI is a prescription medicine used with other antiviral medicines to treat adults with chronic (lasting a long time) hepatitis C virus (HCV):
It is not known if SOVALDI is safe and effective in children under 3 years of age with HCV genotype 2 or 3 infection, or with HCV genotype 1 or 4 infection. It is not known if SOVALDI is safe and effective in people who have had a liver transplant. | ||||
Before taking SOVALDI, tell your healthcare provider about all of your medical conditions, including if you:
Keep a list of your medicines to show your healthcare provider and pharmacist.
| ||||
How should I take SOVALDI?
| ||||
How should I give SOVALDI oral pellets to my child?See the detailed Instructions for Use for information about how to give or take a dose of SOVALDI oral pellets.
| ||||
What are the possible side effects of SOVALDI?SOVALDI can cause serious side effects, including:
| ||||
|
|
| ||
| The most common side effects of SOVALDI when used in combination with ribavirin include: | ||||
|
| |||
| The most common side effects of SOVALDI when used in combination with peginterferon alfa and ribavirin include: | ||||
|
|
| ||
| These are not all the possible side effects of SOVALDI. For more information, ask your healthcare provider or pharmacist. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. | ||||
How should I store SOVALDI?
| ||||
| General information about the safe and effective use of SOVALDI. Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use SOVALDI for a condition for which it was not prescribed. Do not give SOVALDI to other people, even if they have the same symptoms you have. It may harm them. You can ask your healthcare provider or pharmacist for information about SOVALDI that is written for health professionals. For more information, call 1-800-445-3235 or go to www.SOVALDI.com. | ||||
| What are the ingredients in SOVALDI?Active ingredient: sofosbuvir Inactive ingredients, Tablets: colloidal silicon dioxide, croscarmellose sodium, magnesium stearate, mannitol, and microcrystalline cellulose. The tablet film-coat contains polyethylene glycol, polyvinyl alcohol, talc, titanium dioxide, and yellow iron oxide. Inactive ingredients, Oral Pellets: amino methacrylate copolymer, colloidal silicon dioxide, croscarmellose sodium, hydroxypropyl cellulose, hypromellose, lactose monohydrate, microcrystalline cellulose, polyethylene glycol, silicon dioxide, sodium lauryl sulfate, sodium stearyl fumarate, stearic acid, and talc. Gilead Sciences, Inc., Foster City, CA 94404 For more information, call 1-800-445-3235 or go to www.SOVALDI.com. SOVALDI is a trademark of Gilead Sciences, Inc., or its related companies. All other trademarks referenced herein are the property of their respective owners. ©2024 Gilead Sciences, Inc. All rights reserved. 204671-GS-011 | ||||
Instructions For Use Section
SOVALDI® (soh-VAHL-dee)(sofosbuvir)pellets, for oral use
Read the Patient Information that comes with SOVALDI oral pellets for important information about SOVALDI.
This Instructions for Use contains information on how to take SOVALDI oral pellets. Be sure you understand and follow the instructions. If you have any questions, ask your healthcare provider or pharmacist.
Important Information You Need to Know Before Taking SOVALDI oral pellets
- For oral use only (take by mouth with or without food).
- Do not open the SOVALDI oral pellet packet(s) until ready to use.
- SOVALDI oral pellets are white to off-white pellets supplied as single-use packets in cartons. Each carton contains 28 packets.
- Do not use SOVALDI oral pellets if the carton tamper-evident seal, or the pellets packet seal, is broken or damaged.
| Preparing a dose of SOVALDI oral pellets to be taken with food: |
Before you prepare a dose of SOVALDI oral pellets to be taken with food, gather the following supplies:
- Daily SOVALDI oral pellet packet(s), as prescribed by your healthcare provider
- One or more spoonfuls of non-acidic soft food such as pudding, chocolate syrup, mashed potato, or ice cream
- Bowl
- Spoon
- Scissors (optional)
Step 1: Add one or more spoonfuls of non-acidic soft food to the bowl first.
| Step 2: Hold the SOVALDI oral pellets packet with the cut line on top (see Figure A). | Step 3: Shake the packet gently to settle the pellets to the bottom of the packet (see Figure B). | |
|
| |
| Figure A | Figure B |
Step 4: Cut the packet along the cut line with scissors (see Figure C), or fold the packet back at the tear line (see Figure D) and tear open (see Figure E).
|
|
|
|
| Figure C | Figure D | Figure E |
Step 5: Carefully pour the entire contents of the prescribed number of SOVALDI oral pellet packet(s) onto the food in the bowl and gently mix with a spoon (see Figure F). Make sure that no SOVALDI oral pellets remain in the packet(s).
|
| Figure F |
Step 6: Take the SOVALDI oral pellets and food mixture within 30 minutes without chewing to avoid a bitter taste. Ensure all of the SOVALDI oral pellets are taken.
| Preparing a dose of SOVALDI oral pellets to be taken without food: |
Before you prepare a dose of SOVALDI oral pellets to be taken without food, gather the following supplies:
- Daily SOVALDI oral pellet packet(s), as prescribed by your healthcare provider
- Scissors (optional)
- Water (optional)
| Step 1: Hold the SOVALDI oral pellets packet with the cut line on top (see Figure G). | Step 2: Shake the packet gently to settle the pellets to the bottom of the packet (see Figure H). | |
|
| |
| Figure G | Figure H |
Step 3: Cut the packet along the cut line with scissors (see Figure I), or fold the packet back at the tear line (see Figure J) and tear open (see Figure K).
|  |
|
|
| Figure I | Figure J | Figure K |
Step 4: Pour the entire contents of the SOVALDI oral pellets packet directly in the mouth and swallow without chewing to avoid a bitter taste (see Figure L). Water may be taken after swallowing the pellets, if needed. Make sure that no SOVALDI oral pellets remain in the packet. If your healthcare provider prescribed more than one SOVALDI oral pellets packet, repeat Steps 1 through 4.
|
| Figure L |
Storing SOVALDI oral pellets
- Store SOVALDI pellets below 86°F (30°C).
- Keep SOVALDI oral pellets and all medicines out of the reach of children.
Disposing of SOVALDI oral pellets
- Throw away any unused portion. Do not store and reuse any leftover SOVALDI mixture (pellets mixed with food).
For more information, call 1-800-445-3235 or go to www.SOVALDI.com.
Manufactured for and distributed by: Gilead Sciences, Inc., Foster City, CA 94404SOVALDI is a trademark of Gilead Sciences, Inc., or its related companies.
© 2024 Gilead Sciences, Inc. All rights reserved.204671-GS-011
This Instructions for Use has been approved by the U.S. Food and Drug Administration.Issued: March 2020
 Elbasvir-Grazoprevir Zepatier
Elbasvir-Grazoprevir Zepatier Glecaprevir-Pibrentasvir Mavyret
Glecaprevir-Pibrentasvir Mavyret Ledipasvir-Sofosbuvir Harvoni
Ledipasvir-Sofosbuvir Harvoni Ribavirin Copegus, Rebetol, Ribasphere
Ribavirin Copegus, Rebetol, Ribasphere Sofosbuvir Sovaldi
Sofosbuvir Sovaldi Sofosbuvir-Velpatasvir Epclusa
Sofosbuvir-Velpatasvir Epclusa Sofosbuvir-Velpatasvir-Voxilaprevir Vosevi
Sofosbuvir-Velpatasvir-Voxilaprevir Vosevi