Among persons with ascites who have been followed for a year, spontaneous bacterial peritonitis (SBP) develops in approximately 10 to 30% and has an estimated in-hospital mortality rate of 20%.[1,2,3] Among persons with cirrhosis, the prevalence of SBP is 1.5 to 3.5% in an outpatient setting and approximately 10% in an inpatient setting. In most instances, SBP results from translocation of bacteria from the intestinal lumen.[4,5,6] Less often, SBP results from bacteremia that originates at a distant site, such as a urinary tract infection. The majority of cases of SBP are caused by gram-negative enteric organisms, such as Escherichia coli and Klebsiella pneumoniae, but in recent years, the proportion of SBP caused by gram-positive cocci, such as Streptococcus pneumoniae, Staphylococcus species, and Enterococcus species, has increased significantly.[1,7,8] Risk factors associated with the development of SBP include cirrhosis, ascitic fluid total protein less than 1 g/dL, total serum bilirubin greater than 2.5 mg/dL, variceal hemorrhage, and a previous episode of SBP.[9,10,11,12] The use of proton pump inhibitors may slightly increase the risk of developing SBP in persons with cirrhosis and ascites; therefore, in this setting, proton pump inhibitors should be prescribed only in persons who have a clear indication.[13]
- Module 3 Overview
Management of Cirrhosis-Related Complications - 0%Lesson 1
Diagnosis and Management of AscitesActivities- 0%Lesson 2
Recognition and Management of Spontaneous Bacterial PeritonitisActivities- 0%Lesson 3
Screening for Varices and Prevention of BleedingLesson 2. Recognition and Management of Spontaneous Bacterial Peritonitis
PDF ShareLast Updated: March 4th, 2024Author:Jennifer Price, MD, PhDJennifer Price, MD, PhD
Professor of Medicine
Director, UCSF Viral Hepatitis Center
Division of GI and Hepatology
University of California, San FranciscoDisclosures:- Grants, Research support to Institution: Abbvie, Genentech, Vir Biotechnology, Zydus Pharmaceuticals
Reviewers:Maria A. Corcorran, MD, MPH,Maria A. Corcorran, MD, MPH
Associate Editor
Assistant Professor
Division of Allergy & Infectious Diseases
University of WashingtonDisclosures: NoneDavid H. Spach, MDDavid H. Spach, MD
Editor-in-Chief
Professor of Medicine
Division of Allergy & Infectious Diseases
University of WashingtonDisclosures: NoneLearning Objective Performance Indicators
- Explain the diagnostic criteria for spontaneous bacterial peritonitis
- Differentiate spontaneous bacterial peritonitis from secondary bacterial peritonitis
- Select appropriate antimicrobial therapy for persons with spontaneous bacterial peritonitis
- List indications for initiating primary and secondary bacterial peritonitis prophylaxis
- Discuss appropriate regimens for primary and secondary bacterial peritonitis prophylaxis
Table of Contents- Recognition and Management of Spontaneous Bacterial Peritonitis
- Background
- Diagnosis of Spontaneous Bacterial Peritonitis
- Treatment of Spontaneous Bacterial Peritonitis
- Indications for Spontaneous Bacterial Peritonitis Prophylaxis
- Regimens for Spontaneous Bacterial Peritonitis Prophylaxis
- Summary Points
- Citations
- Additional References
- Figures
Background
Diagnosis of Spontaneous Bacterial Peritonitis
Indications for Testing
In a person with ascites, the presence of new-onset fever (temperature greater than 37.8°C or 100°F), abdominal pain, hepatic encephalopathy, metabolic acidosis, renal failure, hypotension, diarrhea, paralytic ileus, hypothermia, leukocytosis, or other signs or symptoms of infection should prompt a diagnostic paracentesis for ascitic fluid analysis and culture (Figure 1).[14] Approximately 13% of individuals with SBP present without any symptoms. For persons with cirrhosis and ascites who are admitted to the hospital, approximately 10 to 15% have evidence of SBP.[15] Thus, all persons with cirrhosis and ascites should undergo a diagnostic paracentesis at the time of hospital admission.[14] In addition, paracentesis should be repeated in persons who develop signs or symptoms of infection. There is no need for transfusion of plasma or platelets prior to a diagnostic paracentesis, given the extremely low risk of hemorrhagic complications, except in the setting of disseminated intravascular coagulation or clinically apparent hyperfibrinolysis.[16,17,18]
Diagnostic Criteria and Classification of SBP
Spontaneous bacterial peritonitis refers to infection of the ascitic fluid, as evidenced by an ascitic fluid absolute polymorphonuclear leukocyte (PMN) count of at least 250 cells/mm3 (0.25 × 109/L), with or without a positive ascitic fluid culture, in the absence of an intra-abdominal surgically treatable source of infection.[1,14] Culture-negative neutrocytic ascites refers to individuals who have an ascitic fluid PMN count of at least 250 cells/mm3 (0.25 × 109/L) in combination with a negative bacterial culture—in the absence of another explanation for an elevated PMN count (e.g., pancreatitis, peritoneal carcinomatosis, or peritoneal tuberculosis) or recent receipt of antimicrobial therapy.[19] Obtaining ascitic fluid for diagnostic testing should be performed before treatment is initiated, as even a single dose of broad-spectrum antibiotics can lead to no growth on bacterial culture in 86% of cases. Approximately 1 mL of ascitic fluid should be injected directly into a purple top EDTA tube for the cell count and differential analysis. In the case of a traumatic paracentesis, with the entry of blood into the ascitic fluid (typically ascitic red cells greater than 10,000 cells/mm3), the PMN count should be corrected by subtracting one PMN from the absolute PMN count for every 250 red cells/mm3.
Bacterial Culture
Prior to administering antibiotics, ascitic fluid (at least 10 mL) should be obtained and then directly inoculated into a blood culture bottle at the bedside, instead of sending the fluid to the laboratory in a syringe or container, since immediate inoculation improves the yield on bacterial culture from approximately 65 to 90% when the ascitic fluid cell count is at least 250 cells/mm3 (0.25 × 109/L).[14,20,21] Separate and simultaneous blood cultures should also be obtained, as up to 50% of persons with SBP have concomitant bacteremia.
Distinguishing Spontaneous from Secondary Bacterial Peritonitis
Secondary peritonitis refers to infection of the ascitic fluid caused by an intraabdominal surgically treatable source and can present similar to SBP. It is important to distinguish SBP from secondary bacterial peritonitis because of the critical need to determine whether surgical intervention is needed. Specifically, mortality approaches 100% in persons with secondary bacterial peritonitis who receive treatment with antibiotics alone (without surgery); mortality is approximately 80% in persons with cirrhosis and SBP who undergo an unnecessary exploratory laparotomy.[22,23,24]
- Recommended Diagnostic Tests: Diagnostic tests may help distinguish SBP from secondary bacterial peritonitis due to a perforated viscus or a loculated abscess.[22,23] Characteristically, with secondary bacterial peritonitis, the fluid PMN count is at least 250 cells/mm3 (usually greater than several thousand) and multiple organisms, including fungi, are identified on Gram’s stain and isolated in culture.
- Diagnostic Criteria: Laboratory diagnostic criteria for secondary bacterial peritonitis include at least two of the following: ascitic fluid protein greater than 1 g/dL, lactate dehydrogenase higher than the upper limit of normal for serum, or glucose less than 50 mg/dL.[23] In addition, ascitic fluid carcinoembryonic antigen greater than 5 ng/mL and alkaline phosphatase greater than 240 U/L have been shown to be associated with gut perforation.[25]
- Treatment Course: After 48 hours of appropriate antibiotic therapy, the ascitic fluid PMN count should decrease with SBP (typically at least 25% lower than the pretreatment level), but with secondary bacterial peritonitis, the PMN count may increase. In addition, persistent signs and symptoms of peritonitis despite appropriate therapy for SBP should prompt an evaluation for secondary bacterial peritonitis.[26]
- Management of Secondary Bacterial Peritonitis: Individuals who meet criteria for secondary bacterial peritonitis—or in whom there is a high suspicion for secondary bacterial peritonitis—should undergo immediate abdominal imaging, and emergent laparotomy should be considered if perforation or a surgically treatable site of infection is identified or strongly suspected.[22,23]
Other Diagnostic Tests on Ascitic Fluid
For an initial diagnostic paracentesis, other tests should be performed as clinically warranted on the remaining ascitic fluid. These tests can be submitted to the laboratory using a red top tube and may include albumin, total protein, glucose, lactate dehydrogenase, amylase, and bilirubin. Most of these additional diagnostic tests will not need repeating for persons with a prior paracentesis, especially a recent paracentesis. The following summarizes some of the key findings with additional diagnostic tests.
- A serum-ascites albumin gradient (SAAG) of 1.1 g/dL or greater is consistent with portal hypertension.[14]
- A total protein level of less than 1.0 g/dL is associated with an increased risk of spontaneous bacterial peritonitis.[11]
- Elevated total protein, low glucose concentration, and elevated lactate dehydrogenase ascitic values are seen in the setting of secondary bacterial peritonitis.[22]
- Elevated ascitic amylase can occur in persons who have pancreatitis and in those with gut perforation. Biliary leakage into the peritoneum can be associated with increased ascitic fluid bilirubin concentration.
Treatment of Spontaneous Bacterial Peritonitis
Criteria for Treatment
Individuals with suspected spontaneous bacterial peritonitis (SBP) and ascitic fluid PMN greater than or equal to 250 cells/mm3 (0.25 × 109/L) should promptly receive empiric antibiotic therapy. Further, persons with culture-negative neutrocytic ascites have similar mortality rates as persons with culture-positive spontaneous bacterial peritonitis and benefit from antibiotic treatment, which should not be delayed while awaiting bacterial culture results (Figure 2).[14,19] Antimicrobial therapy should be given as soon as ascitic fluid has been obtained for culture and should not be delayed while awaiting culture results.
Empiric Therapy in Persons with Non-Neutrocytic Bacterascites
An asymptomatic individual with monomicrobial non-neutrocytic bacterascites (normal ascitic PMN count defined as less than 250 cells/mm3 and positive ascitic fluid culture) does not require immediate antibiotic treatment since bacterascites may represent transient colonization. In this situation, when the culture growth is discovered, the person should undergo a follow-up paracentesis after 48 hours (or if they develop symptoms) to repeat the cell count and culture results to ensure that bacterascites has not progressed to true SBP. Any person with cirrhosis who has a positive ascitic fluid culture and concerning signs or symptoms that may indicate infection, such as fever (temperature greater than 37.8°C or 100°F), abdominal pain, or unexplained hepatic encephalopathy, should receive empiric antibiotic treatment for spontaneous bacterial peritonitis, regardless of ascitic fluid PMN count.
Treatment Regimens
Broad-spectrum antibiotic therapy is recommended for treatment of proven or suspected SBP and may be narrowed when susceptibility results become available.[1,14] Studies have demonstrated resistance rates of approximately 30% in gram-negative infections to fluoroquinolones and trimethoprim-sulfamethoxazole, with particularly high rates in persons who have received fluoroquinolone prophylaxis; on the other hand, more than 90% of isolates in persons who have received fluoroquinolone prophylaxis still remain susceptible to cefotaxime. Therefore, third-generation cephalosporins, such as cefotaxime (2 grams every 8 hours for 5 days) and ceftriaxone (1 gram every 12 hours or 2 grams every 24 hours for 5 days), are the first-line agents for empirical treatment of community-acquired SBP.[14] Extended-spectrum antibiotics, such as piperacillin-tazobactam or carbapenems, may even be considered in nosocomial cases or in patients who are critically ill. In this setting, meropenem should be used instead of piperacillin-tazobactam in persons with current or recent piperacillin-tazobactam use.[14] In addition, vancomycin should be added to the broad-spectrum regimens in persons who have prior SBP or a positive surveillance culture for methicillin-resistant Staphylococcus aureus (MRSA).[14] Further, in this situation, persons with prior SBP or a positive surveillance swab for vancomycin-resistant enterococcus should receive daptomycin instead of vancomycin.[14]
Adjunctive Intravenous Albumin
In a randomized, controlled study involving persons with cirrhosis and SBP, the use of intravenous albumin (1.5 g/kg given within 6 hours of enrollment and repeated as a 1.0 g/kg dose on day 3) as an adjunctive to cefotaxime was shown to decrease in-hospital mortality when compared with use of cefotaxime alone (29% versus 10%).[27] In addition, those treated with albumin had a reduction in the development of renal impairment (10% versus 33%) (Figure 3). Intravenous albumin should be given for persons who have any of the following: a serum creatinine greater than 1 mg/dL, blood urea nitrogen greater than 30 mg/dL, or total bilirubin greater than 4 mg/dL.[28]
Follow-up Diagnostic Paracentesis
Due to increasing failures of initial antibiotic therapy, follow-up ascitic fluid analysis 48 hours after initiating antibiotic therapy is recommended.[14] If the ascitic fluid PMN count has not declined by at least 25% after two days of antibiotic therapy, then the antibiotic coverage needs to be broadened to cover resistant organisms, and secondary bacterial peritonitis needs to be considered. Repeat paracentesis may not be necessary if the patient is clinically improving and an organism is isolated from the initial paracentesis that is susceptible to the antibiotic administered.[14] Individuals with secondary bacterial peritonitis should undergo surgical intervention of the perforated viscus or drainage of the abscess and should be treated with broad-spectrum antibiotics, such as third-generation cephalosporins, with the addition of an antimicrobial agent that has good anaerobic coverage, such as metronidazole.
Indications for Spontaneous Bacterial Peritonitis Prophylaxis
Most episodes of SBP are thought to result from bacterial translocation from the gut.[4,5,6] Given the risk of resistance and alteration of gut flora, this long-term antibiotic prophylaxis should be reserved only for persons at high risk of developing SBP. Identified risk factors for the development of SBP include ascitic fluid total protein less than 1 g/dL, a history of gastrointestinal hemorrhage, and a previous history of SBP.
Secondary Prophylaxis of Spontaneous Bacterial Peritonitis
After a primary episode of SBP, the recurrence rate at one year is approximately 70%, with a 1-year overall survival rate of 30 to 50% among persons who do not receive antibiotic prophylaxis. Secondary antibiotic prophylaxis in a person with cirrhosis who has a prior history of SBP reduces the risk of SBP recurrence from 68% to 20%. Accordingly, most experts recommend daily long-term antimicrobial prophylaxis for persons with a history of one or more episodes of SBP (Figure 4).[14]
Primary Prophylaxis of Spontaneous Bacterial Peritonitis
Persons with cirrhosis who have low-protein ascites (less than 1.0 g/dL) and either impaired renal or liver function are at increased risk of developing SBP.[14] Although controversy exists regarding the use of prophylactic antibiotics in persons without a prior history of SBP (primary prophylaxis), in one randomized trial, daily oral norfloxacin in persons with more advanced liver disease prevented the development of spontaneous bacterial peritonitis and hepatorenal syndrome and improved survival at 3 months when compared with those who received placebo.[29] The American Association for the Study of Liver Diseases (AASLD) guidance suggests using long-term antibiotic prophylaxis in persons who have ascitic fluid total protein less than 1.5 g/dL and at least one of the following: impaired renal function (serum creatinine greater than or equal to 1.2 mg/dL, blood urea nitrogen greater than or equal to 25 mg/dL, or serum sodium less than or equal to 130 mEq/L), or liver failure (Child-Turcotte-Pugh greater than or equal to 9 points and total bilirubin greater than or equal to 3 mg/dL).[14]
Gastrointestinal Hemorrhage
Between 25% and 65% of persons with cirrhosis and gastrointestinal bleeding will develop a bacterial infection, including spontaneous bacterial peritonitis, in the setting of the bleeding episode.[30] Antibiotic prophylaxis in this setting has been shown to decrease the risk of bacterial infection, the risk of re-bleeding, and overall mortality. In one meta-analysis of five trials, antibiotic prophylaxis in persons with cirrhosis and gastrointestinal bleeding demonstrated a 9% increase in survival.[31] Indeed, the use of prophylactic antibiotics in this setting is thought to have contributed significantly to the reduced mortality in persons with variceal bleeding (from 43% to 15%) over the past two decades.[32] In this situation, the AASLD guidance recommends using a 7-day course of prophylactic antimicrobials.[14]
Regimens for Spontaneous Bacterial Peritonitis Prophylaxis
Primary and Secondary Spontaneous Bacterial Peritonitis Prophylaxis
Several studies have shown that oral norfloxacin 400 mg daily prevents SBP in persons with low-protein ascites and those with a previous history of SBP.[33] Alternative regimens that have been studied include oral double-strength trimethoprim-sulfamethoxazole 5 doses per week or oral ciprofloxacin 750 mg once a week.[34,35] Prolonged use of antibimicrobial prophylaxis in this setting has led to the development of gram-negative bacterial resistance (to fluoroquinolones and trimethoprim-sulfamethoxazole), as well as an increased likelihood of developing gram-positive infections.[36,37] Therefore, prophylaxis should be reserved for persons at high risk of developing SBP, and daily dosing regimens are preferred. Daily long-term dosing with norfloxacin has proved superior to hospital-only administration of norfloxacin in the prevention of the first episode of SBP in persons with cirrhosis who have a serum total bilirubin greater than 2.5 mg/dL or ascitic fluid protein less than or equal to 1.5 g/dL.[38] The preferred prophylaxis regimen has been oral norfloxacin 400 mg daily, but given that norfloxacin is no longer available in the United States, reasonable alternatives include trimethoprim-sulfamethoxazole one double-strength tablet daily, oral ciprofloxacin 500 mg daily, or oral levofloxacin 250 mg daily (Figure 5).[14]
Infection Prophylaxis after Gastrointestinal Hemorrhage
Oral norfloxacin 400 mg twice daily for 7 days has been shown to prevent infection in persons with cirrhosis following gastrointestinal hemorrhage.[39] In addition, intravenous ceftriaxone 1 gram daily for 7 days was shown to be superior to norfloxacin for SBP prophylaxis in a randomized trial that enrolled participants with gastrointestinal hemorrhage and advanced cirrhosis, as defined by two of the following: ascites, severe malnutrition, encephalopathy, or bilirubin greater than 3 mg/dL.[37] In persons with cirrhosis and gastrointestinal hemorrhage, the AASLD guidance recommends using a 7-day course of prophylactic antimicrobials with intravenous ceftriaxone 1 g daily, with the option to switch to oral therapy once bleeding stops and the person has resumed oral intake.[14] Since norfloxacin is no longer available in the United States, many experts instead use oral ciprofloxacin 500 mg twice daily or trimethoprim-sulfamethoxazole, one double-strength tablet twice daily in this setting, to complete the 7-day course. If intravenous ceftriaxone cannot be used due to a severe beta-lactam allergy, intravenous ciprofloxacin 400 mg every 12 hours could be used as the initial prophylaxis regimen during active bleeding.
Summary Points
- New onset of fever, abdominal pain, confusion, or other signs or symptoms of infection in a person with cirrhosis should prompt an evaluation of the ascitic fluid for SBP.
-
Individuals with cirrhosis and ascites who are admitted to the hospital should undergo diagnostic paracentesis to evaluate for SBP, even in the absence of signs or symptoms of infection.
- For a diagnostic paracentesis, ascitic fluid should be sent for cell count and differential analysis and should be directly inoculated into blood culture bottles at the bedside.
- Individuals with ascitic fluid PMN count greater than or equal to 250 cells/mm3 meet criteria for a presumptive diagnosis of SBP and should be treated with antibiotic therapy.
- Any person with cirrhosis and ascites who has signs or symptoms concerning for SBP should be treated with antibiotic therapy regardless of ascitic fluid PMN count.
- Recommended therapy for community-acquired SBP consists of intravenous cefotaxime 2 grams every 8 hours (or a similar third-generation cephalosporin) for a duration of 5-7 days.
- Antibiotic prophylaxis for community-acquired SBP should be given to persons with cirrhosis with a prior history of SBP or acute gastrointestinal bleeding, and should be considered in persons without a history of SBP who have renal and/or hepatic dysfunction—if the ascitic fluid total protein is less than 1.5 g/dL.
- Recommended regimens for primary and secondary SBP prophylaxis consist of oral ciprofloxacin (500 mg daily) or trimethoprim-sulfamethoxazole (one double-strength tablet daily). Daily dosing is preferred over intermittent dosing due to the increased risk of developing antimicrobial resistance with intermittent dosing.
- For persons with cirrhosis and acute gastrointestinal hemorrhage, intravenous ceftriaxone 1 gram daily is recommended for a total duration of 7 days. Alternatively, after control of bleeding and resumption of oral intake, ceftriaxone can be transitioned to oral ciprofloxacin 500 mg twice daily or oral trimethoprim-sulfamethoxazole, one double-strength tablet twice daily, to complete the 7-day course.
Citations
- 1.Dever JB, Sheikh MY. Review article: spontaneous bacterial peritonitis--bacteriology, diagnosis, treatment, risk factors and prevention. Aliment Pharmacol Ther. 2015;41:1116-31.[PubMed Abstract] -
- 2.Alaniz C, Regal RE. Spontaneous bacterial peritonitis: a review of treatment options. P T. 2009;34:204-10.[PubMed Abstract] -
- 3.Hurwich DB, Lindor KD, Hay JE, Gross JB Jr, Kaese D, Rakela J. Prevalence of peritonitis and the ascitic fluid protein concentration among chronic liver disease patients. Am J Gastroenterol. 1993;88:1254-7.[PubMed Abstract] -
- 4.Scarpellini E, Valenza V, Gabrielli M, et al. Intestinal permeability in cirrhotic patients with and without spontaneous bacterial peritonitis: is the ring closed? Am J Gastroenterol. 2010;105:323-7.[PubMed Abstract] -
- 5.Guarner C, Soriano G. Bacterial translocation and its consequences in patients with cirrhosis. Eur J Gastroenterol Hepatol. 2005;17:27-31.[PubMed Abstract] -
- 6.Runyon BA, Squier S, Borzio M. Translocation of gut bacteria in rats with cirrhosis to mesenteric lymph nodes partially explains the pathogenesis of spontaneous bacterial peritonitis. J Hepatol. 1994;21:792-6.[PubMed Abstract] -
- 7.Friedrich K, Nüssle S, Rehlen T, Stremmel W, Mischnik A, Eisenbach C. Microbiology and resistance in first episodes of spontaneous bacterial peritonitis: implications for management and prognosis. J Gastroenterol Hepatol. 2016;31:1191-5.[PubMed Abstract] -
- 8.Goel A, Biewald M, Huprikar S, Schiano T, Im GY. A Real-World Evaluation of Repeat Paracentesis-guided Management of Spontaneous Bacterial Peritonitis. J Clin Gastroenterol. 2017;51:278-284.[PubMed Abstract] -
- 9.Andreu M, Sola R, Sitges-Serra A, et al. Risk factors for spontaneous bacterial peritonitis in cirrhotic patients with ascites. Gastroenterology. 1993;104:1133-8.[PubMed Abstract] -
- 10.Llach J, Rimola A, Navasa M, et al. Incidence and predictive factors of first episode of spontaneous bacterial peritonitis in cirrhosis with ascites: relevance of ascitic fluid protein concentration. Hepatology. 1992;16:724-7.[PubMed Abstract] -
- 11.Runyon BA. Low-protein-concentration ascitic fluid is predisposed to spontaneous bacterial peritonitis. Gastroenterology. 1986;91:1343-6.[PubMed Abstract] -
- 12.Titó L, Rimola A, Ginès P, Llach J, Arroyo V, Rodés J. Recurrence of spontaneous bacterial peritonitis in cirrhosis: frequency and predictive factors. Hepatology. 1988;8:27-31.[PubMed Abstract] -
- 13.Khan MA, Kamal S, Khan S, Lee WM, Howden CW. Systematic review and meta-analysis of the possible association between pharmacological gastric acid suppression and spontaneous bacterial peritonitis. Eur J Gastroenterol Hepatol. 2015;27:1327-36.[PubMed Abstract] -
- 14.Biggins SW, Angeli P, Garcia-Tsao G, et al. Diagnosis, Evaluation, and Management of Ascites, Spontaneous Bacterial Peritonitis and Hepatorenal Syndrome: 2021 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2021;74:1014-48.[PubMed Abstract] -
- 15.Borzio M, Salerno F, Piantoni L, et al. Bacterial infection in patients with advanced cirrhosis: a multicentre prospective study. Dig Liver Dis. 2001;33:41-8.[PubMed Abstract] -
- 16.McVay PA, Toy PT. Lack of increased bleeding after paracentesis and thoracentesis in patients with mild coagulation abnormalities. Transfusion. 1991;31:164-71.[PubMed Abstract] -
- 17.Grabau CM, Crago SF, Hoff LK, Simon JA, Melton CA, Ott BJ, Kamath PS. Performance standards for therapeutic abdominal paracentesis. Hepatology. 2004;40:484-8.[PubMed Abstract] -
- 18.Northup PG, Garcia-Pagan JC, Garcia-Tsao G, et al. Vascular Liver Disorders, Portal Vein Thrombosis, and Procedural Bleeding in Patients With Liver Disease: 2020 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2021;73:366-413.[PubMed Abstract] -
- 19.Runyon BA, Hoefs JC. Culture-negative neutrocytic ascites: a variant of spontaneous bacterial peritonitis. Hepatology. 1984;4;1209-11.[PubMed Abstract] -
- 20.Runyon BA, Antillon MR, Akriviadis EA, McHutchinson JG. Bedside inoculation of blood culture bottles is superior to delayed inoculation in the detection of spontaneous bacterial peritonitis. J Clin Microbiol. 1990;28:2811-2.[PubMed Abstract] -
- 21.Sheer TA, Runyon BA. Spontaneous bacterial peritonitis. Dig Dis. 2005;23:39-46.[PubMed Abstract] -
- 22.Soriano G, Castellote J, Alvarez C, et al. Secondary bacterial peritonitis in cirrhosis: a retrospective study of clinical and analytical characteristics, diagnosis and management. J Hepatol. 2010;52:39-44.[PubMed Abstract] -
- 23.Akriviadis EA, Runyon BA. Utility of an algorithm in differentiating spontaneous from secondary bacterial peritonitis. Gastroenterology. 1990;98:127-33.[PubMed Abstract] -
- 24.Garrison RN, Cryer HM, Howard DA, Polk HC Jr. Clarification of risk factors for abdominal operations in patients with hepatic cirrhosis. Ann Surg. 1984;199:648-55.[PubMed Abstract] -
- 25.Wu SS, Lin OS, Chen Y-Y, Hwang KL, Soon MS, Keeffe EB. Ascitic fluid carcinoembryonic antigen and alkaline phosphatase levels for the differentiation of primary from secondary bacterial peritonitis with intestinal perforation. J Hepatol. 2001;34:215-21.[PubMed Abstract] -
- 26.Lu MLR, Agarwal A, Sloan J, Kosmin A. Infected ascites: Distinguishing secondary peritonitis from spontaneous bacterial peritonitis in a cirrhotic patient with classic symptoms. IDCases. 2017;8:29-31.[PubMed Abstract] -
- 27.Sort P, Navasa M, Arroyo V, et al. Effect of intravenous albumin on renal impairment and mortality in patients with cirrhosis and spontaneous bacterial peritonitis. N Engl J Med. 1999;341:403-9.[PubMed Abstract] -
- 28.Sigal SH, Stanca CM, Fernandez J, Arroyo V, Navasa M. Restricted use of albumin for spontaneous bacterial peritonitis. Gut. 2007;56:597-9.[Gut] -
- 29.Fernández J, Navasa M, Planas R, et al. Primary prophylaxis of spontaneous bacterial peritonitis delays hepatorenal syndrome and improves survival in cirrhosis. Gastroenterology. 2007;133:818-24.[PubMed Abstract] -
- 30.Deschenes M, Villenueve JP. Risk factors for the development of bacterial infections in hospitalized patients with cirrhosis. Am J Gastroenterol. 1999;94:2193-7.[PubMed Abstract] -
- 31.Bernard B, Grange JD, Khac EN, Amiot X, Opolon P, Poynard T. Antibiotic prophylaxis for the prevention of bacterial infections in cirrhotic patients with gastrointestinal bleeding: a meta-analysis. Hepatology. 1999;29:1655-61.[PubMed Abstract] -
- 32.Carbonnell N, Pauwels A, Serfaty L, Fourdan O, Levy VG, Poupon R. Improved survival after variceal bleeding in patients with cirrhosis over the past two decades. Hepatology. 2004;40: 652-9.[PubMed Abstract] -
- 33.Ginés P, Rimola A, Planas R, et al. Norfloxacin prevents spontaneous bacterial peritonitis recurrence in cirrhosis: results of a double-blind, placebo-controlled trial. Hepatology. 1990;12:716-24.[PubMed Abstract] -
- 34.Singh N, Gayowski T, Yu VL, Wagener MM. Trimethoprim-sulfamethoxazole for the prevention of spontaneous bacterial peritonitis in cirrhosis: a randomized trial. Ann Intern Med. 1995;122:595-8.[PubMed Abstract] -
- 35.Rolachon A, Cordier L, Bacq Y, et al. Ciprofloxacin and long-term prevention of spontaneous bacterial peritonitis: results of a prospective controlled trial. Hepatology. 1995;22:1171-4.[PubMed Abstract] -
- 36.Campillo B, Dupeyron C, Richardet JP, Mangeney N, Leluan G. Epidemiology of severe hospital acquired infections in patients with liver cirrhosis: effect of long-term administration of norfloxacin. Clin Infect Dis. 1998;26:1066-70.[PubMed Abstract] -
- 37.Fernández J, Ruiz del Arbol L, Gomez C, et al. Norfloxacin vs ceftriaxone in the prophylaxis of infections in patients with advanced cirrhosis and hemorrhage. Gastroenterology. 2006;131:1049-56.[PubMed Abstract] -
- 38.Novella M, Sola R, Soriano G, et al. Continuous versus inpatient prophylaxis of the first episode of spontaneous bacterial peritonitis with norfloxacin. Hepatology. 1997;25:532-6.[PubMed Abstract] -
- 39.Soriano G, Guarner C, Tomas A, et al. Norfloxacin prevents bacterial infection in cirrhotics with gastrointestinal hemorrhage. Gastroenterology. 1992;103;1267-72.[PubMed Abstract] -
Additional References
- Baskol M, Gursoy S, Baskol G, Ozbakir O, Guven K, Yucesoy M. Five days of ceftriaxone to treat culture negative neutrocytic ascites in cirrhotic patients. J Clin Gastroenterol. 2003;37:403-5.[PubMed Abstract] -
- Fernández J, Navasa M, Gómez J, Colmenero J, Vila J, Arroyo V, Rodés J. Bacterial infections in cirrhosis: epidemiological changes with invasive procedures and norfloxacin prophlaxis. Hepatology. 2002;35:140-8.[PubMed Abstract] -
- França A, Giordano HM, Sevá-Pereira T, Soares EC. Five days of ceftriaxone to treat spontaneous bacterial peritonitis in cirrhotic patients. J Gastroenterol. 2002;37:119-22.[PubMed Abstract] -
- França AV, De Souza JB, Silva CM, Soares EC. Long-term prognosis of cirrhosis after spontaneous bacterial peritonitis treated with ceftriaxone. J Clin Gastroenterol. 2001;33:295-8.[PubMed Abstract] -
- Garcia-Tsao G. Current Management of the Complications of Cirrhosis and Portal Hypertension: Variceal Hemorrhage, Ascites, and Spontaneous Bacterial Peritonitis. Dig Dis. 2016;34:382-6.[PubMed Abstract] -
- Garcia-Tsao G. Current management of the complications of cirrhosis and portal hypertension: variceal hemorrhage, ascites, and spontaneous bacterial peritonitis. Gastroenterology. 2001;120:726-48.[PubMed Abstract] -
- Rimola A, Garcia-Tsao G, Navasa M, Piddock LJ, Planas R, Bernard B, Inadomi JM. Diagnosis, treatment and prophylaxis of spontaneous bacterial peritonitis: a consensus statement. International Ascites Club. J Hepatol. 2000;32:142-53.[PubMed Abstract] -
- Rimola A, Salmerón, Clemente G, et al. Two different dosages of cefotaxime in the treatment of spontaneous bacterial peritonitis in cirrhosis: results of a prospective, randomized, multicenter study. Hepatology 1995; 21:674-9.[PubMed Abstract] -
- Runyon BA, McHutchinson JG, Antillon MR, Akriviadis EA, Montano A. Short-course versus long-course antibiotic treatment of spontaneous bacterial peritonitis: a randomized controlled trial of 100 patients. Gastroenterology. 1991;100:1737-42.[PubMed Abstract] -
- Runyon BA. Management of adult patients with ascites due to cirrhosis: an update. Hepatology. 2009;49:2087-107.[PubMed Abstract] -
- Tandon P, Garcia-Tsao G. Bacterial infections, sepsis, and multiorgan failure in cirrhosis. Semin Liver Dis. 2008;28:26-42.[PubMed Abstract] -
- Terg R, Cobas S, Fassio E, et al. Oral ciprofloxacin after a short course of intravenous ciprofloxacin in the treatment of spontaneous bacterial peritonitis: results of a multicenter, randomized study. J Hepatol. 2000;33:564-9.[PubMed Abstract] -
Figures
 Figure 1. Indications for Performing Diagnostic ParacentesisSource: Biggins SW, Angeli P, Garcia-Tsao G, et al. Diagnosis, Evaluation, and Management of Ascites, Spontaneous Bacterial Peritonitis and Hepatorenal Syndrome: 2021 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2021;74:1014-48.
Figure 1. Indications for Performing Diagnostic ParacentesisSource: Biggins SW, Angeli P, Garcia-Tsao G, et al. Diagnosis, Evaluation, and Management of Ascites, Spontaneous Bacterial Peritonitis and Hepatorenal Syndrome: 2021 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2021;74:1014-48.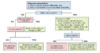 Figure 2. Approach to the Diagnosis and Treatment of Spontaneous Bacterial Peritonitis (SBP)This algorithm provides a general approach to the diagnosis and management of persons with possible spontaneous bacterial peritonitis (SBP). The clinician should suspect SBP with any of the following: (1) inadequate response to antibiotics, (2) more than one organism is isolated from culture, or (3) at least two of the following ascitic fluid values are present—protein greater than 1 g/dL, LDH greater than ULN serum levels, and glucose less than 50 mg/dL. If secondary bacterial peritonitis is suspected, appropriate imaging should be obtained, antibiotic coverage broadened to include anaerobes, and laparotomy considered.Source: Biggins SW, Angeli P, Garcia-Tsao G, et al. Diagnosis, Evaluation, and Management of Ascites, Spontaneous Bacterial Peritonitis and Hepatorenal Syndrome: 2021 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2021;74:1014-48.
Figure 2. Approach to the Diagnosis and Treatment of Spontaneous Bacterial Peritonitis (SBP)This algorithm provides a general approach to the diagnosis and management of persons with possible spontaneous bacterial peritonitis (SBP). The clinician should suspect SBP with any of the following: (1) inadequate response to antibiotics, (2) more than one organism is isolated from culture, or (3) at least two of the following ascitic fluid values are present—protein greater than 1 g/dL, LDH greater than ULN serum levels, and glucose less than 50 mg/dL. If secondary bacterial peritonitis is suspected, appropriate imaging should be obtained, antibiotic coverage broadened to include anaerobes, and laparotomy considered.Source: Biggins SW, Angeli P, Garcia-Tsao G, et al. Diagnosis, Evaluation, and Management of Ascites, Spontaneous Bacterial Peritonitis and Hepatorenal Syndrome: 2021 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2021;74:1014-48. Figure 3. Treatment with IV Albumin plus Cefotaxime in Adults with Spontaneous Bacterial PeritonitisThis graphic shows that the addition of albumin to cefotaxime clearly prevented renal impairment and improved mortality when compared with cefotaxime alone. P values: renal impairment p=0.002, in-hospital mortality p=0.01, 3 month mortality p=0.03Source: Sort P, Navasa M, Arroyo V, et al. Effect of intravenous albumin on renal impairment and mortality in patients with cirrhosis and spontaneous bacterial peritonitis. N Engl J Med. 1999;341:403-9.
Figure 3. Treatment with IV Albumin plus Cefotaxime in Adults with Spontaneous Bacterial PeritonitisThis graphic shows that the addition of albumin to cefotaxime clearly prevented renal impairment and improved mortality when compared with cefotaxime alone. P values: renal impairment p=0.002, in-hospital mortality p=0.01, 3 month mortality p=0.03Source: Sort P, Navasa M, Arroyo V, et al. Effect of intravenous albumin on renal impairment and mortality in patients with cirrhosis and spontaneous bacterial peritonitis. N Engl J Med. 1999;341:403-9. Figure 4. Indications for Spontaneous Bacterial Peritonitis (SBP) ProphylaxisSource: Biggins SW, Angeli P, Garcia-Tsao G, et al. Diagnosis, Evaluation, and Management of Ascites, Spontaneous Bacterial Peritonitis and Hepatorenal Syndrome: 2021 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2021;74:1014-48.
Figure 4. Indications for Spontaneous Bacterial Peritonitis (SBP) ProphylaxisSource: Biggins SW, Angeli P, Garcia-Tsao G, et al. Diagnosis, Evaluation, and Management of Ascites, Spontaneous Bacterial Peritonitis and Hepatorenal Syndrome: 2021 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2021;74:1014-48. Figure 5. Prophylaxis for Spontaneous Bacterial Peritonitis (SBP)This table is adapted from recommendations in the 2021 AASLD Guidelines on the
Figure 5. Prophylaxis for Spontaneous Bacterial Peritonitis (SBP)This table is adapted from recommendations in the 2021 AASLD Guidelines on theDiagnosis, Evaluation, and Management of Ascites, Spontaneous Bacterial Peritonitis and Hepatorenal Syndrome.
Source: Biggins SW, Angeli P, Garcia-Tsao G, et al. Diagnosis, Evaluation, and Management of Ascites, Spontaneous Bacterial Peritonitis and Hepatorenal Syndrome: 2021 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2021;74:1014-48.Share by e-mail
Check
-On-
Learning
QuestionsThe Check-on-Learning Questions are short and topic related. They are meant to help you stay on track throughout each lesson and check your understanding of key concepts.You must be signed in to customize your interaction with these questions.
- 0%Lesson 2
Since you've received 80% or better on this quiz, you may claim continuing education credit.
You seem to have a popup blocker enabled. If you want to skip this dialog please Always allow popup windows for the online course.
Account Registration Benefits:
- Track your progress on the lessons
- Earn free CNE/CME/CE
- Earn Certificates of Completion
- Access to other free IDEA curricula
Create a free account to get started









