Substance use disorders are common in the United States, with 2022 data indicating that nearly 1 in 4 (24.9%) of persons 12 years of age or older used an illicit drug in the past year (Figure 1).[1] The availability of highly effective direct-acting antiviral (DAA) medications has radically changed the assessment and consideration of substance use in hepatitis C virus (HCV) treatment decisions. The AASLD-IDSA HCV Guidance states that recent or active injection drug use or alcohol use is not a contraindication to HCV treatment and requirements for pretreatment screening for illicit drug or alcohol use should be discontinued.[2] Nevertheless, for persons with chronic HCV infection, substance use, either past or present, which encompasses the use of opioids, amphetamines, cannabis, cocaine, alcohol, and other drugs, may still be relevant to the individual's overall health and medication access. The following discussion will address the potential impact of substance use on HCV disease and HCV treatment and provide current recommendations for the treatment of HCV in persons with a substance use disorder.
- Module 6 Overview
Treatment of Key Populations and Unique Situations - 0%Lesson 1
Treatment of HCV in Persons with HIV CoinfectionActivities- 0%Lesson 2
Treatment of HCV in Persons with Renal ImpairmentActivities- 0%Lesson 3
Treatment of HCV in Persons with Substance UseActivities- 0%Lesson 4
Treatment of HCV in a Correctional SettingActivities- 0%Lesson 5
Management of Health Care Personnel Exposed to HCVActivities- 0%Lesson 6
Perinatal HCV TransmissionActivitiesLesson 3. Treatment of HCV in Persons with Substance Use
PDF ShareLast Updated: January 25th, 2024Authors:Maria A. Corcorran, MD, MPH,Maria A. Corcorran, MD, MPH
Associate Editor
Assistant Professor
Division of Allergy & Infectious Diseases
University of WashingtonDisclosures: NoneDavid H. Spach, MDDavid H. Spach, MD
Editor-in-Chief
Professor of Medicine
Division of Allergy & Infectious Diseases
University of WashingtonDisclosures: NoneReviewer:H. Nina Kim, MD, MScH. Nina Kim, MD, MSc
Associate Editor
Professor of Medicine
Division of Allergy & Infectious Diseases
University of WashingtonDisclosures: NoneLearning Objective Performance Indicators
- Discuss the prevalence of substance use among persons in the United States
- Describe HCV treatment responses in persons with substance use disorders compared with those who do not have substance use disorders
- Summarize HCV treatment eligibility in persons who have a past or current substance use disorder
- List support services and management strategies for persons with substance use disorders who have chronic HCV infection
- Explain the concept of HCV reinfection in persons who inject drugs
Table of Contents- Treatment of HCV in Persons with Substance Use
- Background
- Approach to HCV Treatment in Persons with Substance Use
- HCV Treatment in Persons with Opioid Use
- Impact of Opioid Use on Natural History of HCV
- Impact of Treating People with Active Injection Drug Use on HCV Transmission
- Impact of Opioid Use and HCV Treatment Adherence
- HCV Treatment Outcomes with DAA Therapy in PWID
- Pretreatment Requirements
- Management Strategies
- Potential Reinfection with HCV among Persons who Inject Drugs
- HCV Treatment in Persons with Stimulant Use
- HCV Treatment in Persons who Consume Alcohol
- HCV Treatment in Persons who Use Cannabis
- Summary Points
- Citations
- Additional References
- Figures
Background
Approach to HCV Treatment in Persons with Substance Use
HCV Treatment Eligibility
The AASLD-IDSA HCV Guidance recommends treatment for all persons with chronic HCV, except those with a short life expectancy that HCV treatment cannot remediate, liver transplantation, or another directed therapy.[2,3] The general approach to considering initiation of HCV treatment for individuals with a prior history of substance use, including injection drug use, should be the same as in persons with no history of drug use. Persons with substance use disorder, including those with injection drug use or alcohol use should have the same HCV pretreatment screening requirements as those without a substance use disorder.[2]
Abstinence Requirements
Although some payers require 6 months or more of abstinence prior to HCV treatment, studies of both injection drug use and alcohol use have found no impact of duration of abstinence on the likelihood of achieving a sustained virologic response 12 weeks (SVR12) after completing DAA-based therapy.[4] Thus, there is no medical reason to ensure abstinence (for any duration) prior to HCV treatment. Current AASLD-IDSA HCV Guidance recommendations state that current or prior substance use is not a contraindication to HCV treatment.[2]
Impact of Comorbidities in Persons with Substance Use Disorder
In clinical practice, treating persons with an active substance use disorder may be complicated by coexisting social stressors, competing survival needs, and barriers erected by payers. However, clinical experience suggests that, with appropriate infrastructure and patient support, including treatment of substance use disorders, HCV treatment is very feasible in this population.[5,6,7,8,9] Examples of patient support include directly-observed therapy and related approaches, patient navigation, and group treatment models, particularly in substance use disorder treatment settings.[10]
HCV Treatment in Persons with Opioid Use
Impact of Opioid Use on Natural History of HCV
Through the sharing of syringes and other injection equipment, injection opioid use is a major driver of HCV transmission, but opioid use itself, either orally or by injection, does not appear to speed the progression of liver disease in persons with chronic HCV.[11] Opioid analgesic use disorder is also a risk factor for HCV acquisition and transmission, as some individuals transition from oral ingestion of prescribed opioids to use of illicit opioids, which can include injection opioid use.[12,13] Nevertheless, opioid use itself, either orally or by injection, does not appear to impact the natural history of liver disease in persons with chronic HCV.[11]
Impact of Treating People with Active Injection Drug Use on HCV Transmission
Mathematical modeling, even assuming a reinfection rate equal to initial infection rates, has demonstrated that HCV treatment among persons with active injection drug use would result in a significant reduction in HCV transmission.[14,15,16,17] Several studies utilizing mathematical modeling based on DAA regimens concluded that scaling up HCV treatment in people who inject drugs (PWID) would have a major impact in reducing HCV incidence and prevalence in this patient population, even more so in the setting of robust access to sterile injection equipment and medications for opioid use disorder (e.g., buprenorphine-naloxone or methadone).[16] Further, scaling up and widespread treatment of HCV in PWID as a prevention tool, akin to treating HIV to reduce community viral load, has become a more realistic goal with the short-course, well-tolerated oral regimens. Indeed, several efforts focusing on HCV micro-elimination (i.e., elimination of HCV as a public health threat within a defined population) have provided real-world evidence for HCV treatment as prevention, demonstrating significant reductions in HCV incidence resulting from widespread treatment.[18,19,20]
Impact of Opioid Use and HCV Treatment Adherence
Multiple studies that have enrolled persons with active or recent injection drug use have shown excellent adherence with DAA-based HCV therapy (Figure 2).[10,21,22,23,24,25] In particular, persons with opioid use disorder who receive opioid agonist maintenance therapy (e.g., methadone, buprenorphine, or buprenorphine-naloxone) during HCV treatment have excellent rates of adherence, treatment completion, and sustained virologic response (SVR) rates, all comparable to results of other study participants.[26,27]
HCV Treatment Outcomes with DAA Therapy in PWID
The following summarizes several key studies that have analyzed HCV treatment responses with DAA-based therapy in persons who inject drugs or who have previously injected drugs and were receiving opioid agonist therapy. Multiple studies clearly show that DAA-based therapy in persons with past or current injection drug use results in high SVR rates, comparable to those seen in persons who do not use drugs.[21,24]
- Elbasvir-Grazoprevir (C-EDGE CO-STAR): In this phase 3 multinational study (C-EDGE CO-STAR), investigators evaluated HCV treatment with elbasvir-grazoprevir in 301 PWID who had also been receiving opioid agonist therapy (e.g. methadone, buprenorphine, or buprenorphine-naloxone maintenance) for at least 3 months prior to enrollment.[23] Participants with chronic HCV genotypes 1, 4, or 6 were randomized to immediately receive a 12-week course of elbasvir-grazoprevir (immediate treatment group) or to receive the 12-week course of elbasvir-grazoprevir after a 16-week delay (deferred treatment group). On day 1 of the study, 58% of the subjects had a positive urine drug screen (excluding opiate agonist therapy). Overall, when excluding participants who discontinued for non-treatment reasons, 92% in the immediate treatment group and 90% in the deferred treatment group achieved an SVR12; the treatment responses were excellent regardless of cirrhosis status and baseline drug screening results (Figure 3).[23]
- Glecaprevir-Pibrentasvir: In a pooled analysis of 7 phase 3 studies that involved HCV treatment with 8 or 12 weeks of glecaprevir-pibrentasvir, investigators compared SVR12 rates among persons with recent drug use (use in the past 12 months), former drug use (use more than 12 months ago), and no drug use.[28] The SVR12 rates were high among all groups: 93% in persons with recent drug use, 97% in those with former drug use, and greater than 99% in persons who did not use drugs.[28] A similar pooled analysis of 8 phase 2 and 3 trials of glecaprevir-pibrentasvir evaluated treatment outcomes among persons receiving opioid substitution therapy.[29] In the intention-to-treat analysis, SVR12 rates were 96% among persons receiving opioid substitution therapy and 98% in those not receiving opioid substitution therapy.[29] In an analysis of 2 prospective cohort studies in Spain, in the intention-to-treat analysis, the SVR12 rates were 97% among persons who never used drugs, 96% among persons with past drug use, and 85% among persons with recent or active drug use.[30] This slightly lower SVR12 rate among persons with recent or active drug use was felt to be secondary to voluntary discontinuation, as no difference in SVR12 rates was seen when comparing persons who never used drugs (99%), persons with past drug use (100%), and persons with recent or active drug use (100%) in the per-protocol analysis.[30]
- Ledipasvir-Sofosbuvir (ION Trials): In the ION trials, participants with chronic HCV genotype 1 infection received 8, 12, or 24 weeks of ledipasvir-sofosbuvir.[31] Investigators performed a pooled data analysis to compare HCV treatment response in participants receiving opioid substitution therapy during treatment (n = 70) with participants not receiving opioid substitution therapy (n = 1882).[31] The two groups had similar treatment completion, adherence, and SVR12 rates (Figure 4).[31] Among those receiving opioid substitution therapy, 94% (66 of 71) achieved an SVR12.[31] In addition, a pilot trial of ledipasvir-sofosbuvir among 31 persons with active injection drug use randomized participants 1:1 to modified directly-observed treatment (mDOT) or unobserved dosing. All but one participant (in the mDOT arm) completed treatment; 97% achieved an end-of-treatment response and 90% achieved SVR12.[32]
- Sofosbuvir-Based Treatment in Phase 3 Trials: In a pooled analysis of data from phase 3 trials using sofosbuvir-based regimens, investigators compared HCV treatment responses in participants receiving opioid substitution therapy during treatment (n = 194) with participants not receiving opioid substitution therapy (n = 4,549).[27] There were no significant differences in SVR12 rates (94% in those receiving opioid substitution therapy versus 97% for those not receiving opioid substitution therapy).[27]
- Sofosbuvir-Velpatasvir (SIMPLIFY): In this single-arm, open-label, phase 4 study, 103 participants with chronic HCV genotype 1, 2, 3, 4, 5, or 6 infection and recent (within 6 months) injection drug use were treated with a 12-week course of sofosbuvir-velpatasvir.[22] During the treatment course, 59% (61 of 103) participants were receiving opioid substitution therapy, and 74% (76 of 103) had used injection drugs in the month prior to starting treatment.[22] A total of 97% (100 of 103) participants completed treatment; 2 were lost to follow-up, and one person died from an accidental overdose. Overall, 97 (94%) of participants achieved an SVR12.[22]
- Sofosbuvir-Velpatasvir (ASTRAL Trials): In a subset analysis of the phase 3 ASTRAL trials, investigators compared treatment response to sofosbuvir-velpatasvir in persons receiving opioid substitution therapy during treatment (n = 51) compared with those who were not receiving opioid substitution therapy (n = 984).[26] Receipt of opioid substitution therapy did not impact treatment responses; 96% of participants receiving opioid substitution therapy achieved an SVR12.[26]
Pretreatment Requirements
The AASLD-IDSA HCV Guidance does not recommend requiring abstinence from opioids prior to HCV treatment.[2] Indeed, active injection drug use in an individual with chronic HCV is considered by many to be a direct indication for HCV treatment, due to the potential benefit of reducing secondary HCV transmission.[33,34] In contrast to these expert recommendations, some payers may require abstinence from non-prescribed opioids and other recreational substances, but this type of requirement is not evidence-based and not consistent with recommendations in the AASLD-IDSA HCV Guidance.[3] There are three FDA-approved treatments for opioid use disorder—buprenorphine, methadone, and extended-release naltrexone. Although methadone access is limited to designated outpatient treatment programs, the other medications can be prescribed in the context of routine medical care.
Management Strategies
For persons with a past or current history of opioid use disorder, treatment of HCV infection would ideally be performed in a multidisciplinary setting whereby treatment for HCV and opioid use disorder can be jointly addressed.[2] Multiple treatment options exist for opioid use. Agonist maintenance therapy is the most effective known treatment and has been shown to reduce the risk of new HCV infection.[35] Data on the use of DAAs and medications for opioid use disorder (MOUD) indicate comparable SVR rates when comparing persons on MOUD with those who do not use drugs.[22,23,26,27,29,31] Injectable extended-release naltrexone is also approved for opioid dependence, although access can be challenging and uptake can be limited. There are no known clinically significant interactions between opioid agonist therapies or naltrexone and currently approved DAA medications.[36,37] A detailed discussion of opioid agonist therapy is beyond the scope of this topic review. In addition, several studies have evaluated optimal models for HCV treatment within harm reduction and opioid treatment programs.[10,38,39] One large study (HERO study) conducted in eight opioid treatment programs and 15 community health centers randomized 755 individuals to sofosbuvir-velpatasvir plus patient navigation or sofosbuvir-velpatasvir plus modified directly observed therapy (mDOT).[10] Although there was slightly higher adherence to sofosbuvir-velpatasvir in the mDOT arm, there was no difference in SVR12 rates, with 60% and 91% of patients in the mDOT arm and 62% and 93% of patients in the patient navigation arm achieving an SVR12 in the intention-to-treat analysis and per-protocol analysis, respectively.[10]
Potential Reinfection with HCV among Persons who Inject Drugs
Multiple studies have shown a significant risk of HCV reinfection in persons cured with HCV therapy. Thus, it is essential to counsel persons with past or active injection drug use be counseled that they can become reinfected with HCV after achieving an SVR. This risk is significant in persons who inject drugs, but reinfection can also occur through sexual contact, particularly among men who have sex with men (MSM).[40] In one study that clearly evaluated reinfection among treated PWID, the reinfection rate for those reporting ongoing injection after SVR was 5.3/100 person-years.[41] Similarly, in a recent systematic review of 36 studies of HCV reinfection following successful HCV treatment in persons who inject drugs, the overall rate of HCV reinfection was 6.2 per 100 person-years among those who reported recent injection drug use.[42] A similar systematic review and meta-analysis of 41 single-armed observational studies found a pooled rate of reinfection of 4.1 per 100 person-years), with a rate of reinfection of 2.8 per 100 person-years among PWID, 7.4 per 100 person-years among MSM, and 7.2 per 100 person-years among incarcerated individuals.[43] Thus, providing access to counseling for safe injection practices and MOUD is an important component of HCV treatment programs. Detailed guidance on safer injection techniques, such as ensuring a source of sterile syringes and other injection equipment and reviewing possible sources of HCV transmission, such as cottons, cookers, water, alcohol pads, or any syringes used to divide, prepare, or inject drugs, may lessen the risk of reinfection.[44]
HCV Treatment in Persons with Stimulant Use
Impact of Stimulant Use on Natural History of HCV
Injection of cocaine or methamphetamine is another major driver of HCV transmission.[45,46,47] Other routes of administration of stimulants, such as intranasal, may also be associated with HCV transmission.[48] In addition, prolonged stimulant use may result in cardiac and cerebrovascular toxicity.
Pretreatment Requirements
There is no medical requirement for abstinence from stimulant use prior to HCV treatment. Although some payers may require abstinence from methamphetamine prior to starting treatment, any such requirement is not consistent with recommendations in the AASLD-IDSA HCV Guidance.[3]
HCV Treatment Outcomes in Persons with Stimulant Use
Although there are limited data specific to the impact of stimulant use on outcomes of DAA therapy, several large trials evaluating treatment outcomes among persons with opioid use disorder have included sizable proportions of persons with concurrent stimulant use.[10,22] For example, in the HERO study, which was a randomized trial of sofosbuvir-velpatasvir plus modified directly observed therapy versus sofosbuvir-velpatasvir plus patient navigation in 8 opioid treatment programs and 15 community health centers, 32% of individuals with available urine drug screen results at baseline had a test positive for methamphetamine, and 42% were positive for cocaine,[10] In this study, SVR12 rates in the per-protocol analysis mirrored those of persons without substance use or stimulant use, with greater than 90% of participants with or without substance use achieving an SVR12.[10] Similarly, in the SIMPLIFY study, an open-label, single-arm trial of sofosbuvir-velpatasvir for persons with recent injection drug use, 30% reported injection methamphetamine use, and 13% reported injection cocaine use in the past 30 days.[22] In this study, 94% of individuals achieved an SVR12.[22]
Management Strategies
Stimulant use is often more intermittent than opioid or alcohol use but can also be associated with periods of poor adherence to medical care. Pharmacologic options are limited, with multiple current trials underway for both methamphetamine and cocaine dependence.[49] In a double-blind, placebo-controlled, randomized trial conducted from 2012 to 2015, a 12-week course of extended-release naltrexone did not appear to reduce amphetamine use among dependent persons.[] In a similar randomized, controlled trial, a 12-week course of aripiprazole, when given over 12 weeks, did not significantly reduce methamphetamine use.[51] Mirtazapine has demonstrated efficacy in reducing methamphetamine use in two separate trials.[52] Bupropion and modafinil have also demonstrated benefits in small trials.[53,54] A combination of high-dose bupropion plus high-frequency extended-release naltrexone demonstrated benefit in a trial of 403 participants.[55]
HCV Treatment in Persons who Consume Alcohol
Impact of Alcohol Use on Liver Fibrosis in Persons with Chronic HCV
Several studies have shown that heavy alcohol consumption (at least 60 grams/day in men and 40 grams/day in women) accelerates the progression of HCV-related hepatic fibrosis (Figure 5).[56,57] A typical alcoholic drink (12 ounces of beer, 5 ounces of wine, and 1.5 ounces of whiskey) contains 12 grams of alcohol. An estimated one-third of patients with chronic HCV infection have cirrhosis attributable to heavy alcohol consumption.[58] In a study in Alaska, investigators compared outcomes in persons who recovered from HCV with those who had chronic HCV and found heavy alcohol use (at least 50 grams of alcohol daily) was associated with the highest incidence of end-stage liver disease, regardless of whether the individual had recovered from HCV or had chronic HCV infection.[59] In addition, separate studies have shown that liver disease progression may continue among persons who heavily use alcohol even if SVR is achieved with HCV treatment. Taken together, the available data suggest reducing alcohol use is critical to liver health. The effects of low or moderate alcohol consumption on liver health are not well characterized for persons with chronic HCV infection.
HCV Treatment Outcomes Among Persons with Alcohol Use
Most of the studies that have addressed whether alcohol use impacts treatment outcomes were performed in the pre-DAA treatment era and results from these studies were mixed.[9,60,61] In the DAA treatment era, a large observational study out of the Veteran’s Affairs (VA) healthcare system evaluated the impact of alcohol use on HCV DAA-based treatment outcomes.[62] Of the 15,151 persons who initiated DAA therapy and had a documented AUDIT-C score, 68.5% were categorized as abstinent, 22.6% as low-level drinking, and 8.9% as unhealthy drinking. Overall SVR12 rates were high among all persons in the study, regardless of alcohol use, with no statistical difference between HCV genotype or by cirrhosis status (Figure 7).[62] These findings support current recommendations to not exclude persons from HCV treatment based on their alcohol use.
Pretreatment Requirements
Although abstinence from alcohol is strongly encouraged for patients with chronic HCV infection, the AASLD-IDSA HCV Guidance recommends that abstinence from alcohol (or a reduction in alcohol consumption) should not be a requirement prior to HCV DAA treatment.[2] Although some payers may still require abstinence from alcohol prior to HCV treatment, this requirement is not based on guidelines or data.
Management Strategies
Although abstinence from alcohol prior to HCV DAA treatment is no longer required, alcohol consumption is discouraged in patients with chronic HCV infection due to the hepatotoxic effects of alcohol and its acceleration in liver fibrosis. Multiple pharmacologic agents are available for alcohol use disorder, including naltrexone, acamprosate, and topiramate.[63] Among these, the most promising results have been seen with naltrexone, particularly when given as a monthly injection. Brief counseling on alcohol has also shown reductions in use among persons with HCV infection. A multidisciplinary approach, involving personalized addiction care and case management, may provide further benefit in managing alcohol dependence.[9] Following DAA-based therapy, one study that included 123 participants found provider-delivered, alcohol-related counseling during HCV treatment was successful in reducing alcohol consumption patterns both during and after treatment in individuals with harmful alcohol use.[64]
HCV Treatment in Persons who Use Cannabis
There is mixed evidence regarding cannabis use and HCV-related hepatic fibrosis progression.[65,66,67] Two separate longitudinal cohort studies found no association between cannabis use and progression of liver fibrosis among patients coinfected with HCV and HIV.[65,68] In addition, one study found a positive association between cannabis use and good adherence with HCV treatment.[69] Although individuals living with HCV are typically advised to abstain from regular cannabis use, ongoing cannabis use is not considered a contraindication for initiating HCV therapy.
Summary Points
- Active or past substance use, substance use disorder, or injection drug use is not a contraindication to HCV treatment.
-
Treatment of HCV in persons with active injection drug use likely has major public health benefits in terms of reducing secondary HCV transmission.
- It is important to talk to patients about their substance use to best understand how to support them through treatment and lower the risk of reinfection.
- Persons with HCV should be aware that heavy use of alcohol may continue to cause damage to the liver.
- Therapeutic approaches to substance use disorders are generally more effective when a pharmacologic agent is included.
- Care should be taken to ensure that PWID are aware of specific drug use techniques to avoid reinfection, particularly in the ways drugs are divided or prepared for injection.
Citations
- 1.Substance Abuse and Mental Health Services Administration (SAMHSA). (2022). Key substance use and mental health indicators in the United States: Results from the 2022 National Survey on Drug Use and Health (HHS Publication No. PEP23-07-01-006, NSDUH Series H-58). Rockville, MD: Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration.[SAMHSA] -
- 2.AASLD-IDSA. HCV Guidance: Recommendations for testing, management, and treating hepatitis C. When and in whom to initiate HCV therapy.
- 3.AASLD-IDSA. HCV Guidance: Recommendations for testing, management, and treating hepatitis C. Key populations: identification and management of HCV in people who inject drugs.
- 4.Hellard M, Sacks-Davis R, Gold J. Hepatitis C treatment for injection drug users: a review of the available evidence. Clin Infect Dis. 2009;49:561-73.[PubMed Abstract] -
- 5.Backmund M, Meyer K, Von Zielonka M, Eichenlaub D. Treatment of hepatitis C infection in injection drug users. Hepatology. 2001;34:188-93.[PubMed Abstract] -
- 6.Gidding HF, Law MG, Amin J, et al. Hepatitis C treatment outcomes in Australian clinics. Med J Aust. 2012;196:633-7.[PubMed Abstract] -
- 7.Grebely J, Raffa JD, Meagher C, et al. Directly observed therapy for the treatment of hepatitis C virus infection in current and former injection drug users. J Gastroenterol Hepatol. 2007;22:1519-25.[PubMed Abstract] -
- 8.Grebely J, Robaeys G, Bruggmann P, et al. Recommendations for the management of hepatitis C virus infection among people who inject drugs. Int J Drug Policy. 2015;26:1028-38.[PubMed Abstract] -
- 9.Le Lan C, Guillygomarc'h A, Danielou H, et al. A multi-disciplinary approach to treating hepatitis C with interferon and ribavirin in alcohol-dependent patients with ongoing abuse. J Hepatol. 2012;56:334-40.[PubMed Abstract] -
- 10.Litwin AH, Lum PJ, Taylor LE, et al. Patient-centred models of hepatitis C treatment for people who inject drugs: a multicentre, pragmatic randomised trial. Lancet Gastroenterol Hepatol. 2022;7:1112-27.[PubMed Abstract] -
- 11.Brunet L, Moodie EE, Cox J, et al. Opioid use and risk of liver fibrosis in HIV/hepatitis C virus-coinfected patients in Canada. HIV Med. 2016;17:36-45.[PubMed Abstract] -
- 12.Compton WM, Jones CM, Baldwin GT. Relationship between Nonmedical Prescription-Opioid Use and Heroin Use. N Engl J Med. 2016;374:154-63.[PubMed Abstract] -
- 13.Jones CM. Heroin use and heroin use risk behaviors among nonmedical users of prescription opioid pain relievers - United States, 2002-2004 and 2008-2010. Drug Alcohol Depend. 2013;132:95-100.[PubMed Abstract] -
- 14.Ward Z, Platt L, Sweeney S, et al. Impact of current and scaled-up levels of hepatitis C prevention and treatment interventions for people who inject drugs in three UK settings-what is required to achieve the WHO's HCV elimination targets? Addiction. 2018. doi: 10.1111/add.14217.[PubMed Abstract] -
- 15.Martin NK, Vickerman P, Grebely J, et al. Hepatitis C virus treatment for prevention among people who inject drugs: Modeling treatment scale-up in the age of direct-acting antivirals. Hepatology. 2013;58:1598-609.[PubMed Abstract] -
- 16.Martin NK, Hickman M, Hutchinson SJ, Goldberg DJ, Vickerman P. Combination interventions to prevent HCV transmission among people who inject drugs: modeling the impact of antiviral treatment, needle and syringe programs, and opiate substitution therapy. Clin Infect Dis. 2013;57 Suppl 2:S39-45.[PubMed Abstract] -
- 17.Martin NK, Vickerman P, Miners A, Foster GR, Hutchinson SJ, Goldberg DJ, Hickman M. Cost-effectiveness of hepatitis C virus antiviral treatment for injection drug user populations. Hepatology. 2012;55:49-57.[PubMed Abstract] -
- 18.Olafsson S, Fridriksdottir RH, Love TJ, et al. Cascade of care during the first 36 months of the treatment as prevention for hepatitis C (TraP HepC) programme in Iceland: a population-based study. Lancet Gastroenterol Hepatol. 2021;6:628-37.[PubMed Abstract] -
- 19.Huang CF, Dai CY, Wang CW, et al. Therapy as prevention toward HCV elimination in maintenance hemodialysis: a multi-center, prospective cohort study. Clin Kidney J. 2023;16:2429-36.[PubMed Abstract] -
- 20.Hajarizadeh B, Grebely J, Byrne M, et al. Evaluation of hepatitis C treatment-as-prevention within Australian prisons (SToP-C): a prospective cohort study. Lancet Gastroenterol Hepatol. 2021;6:533-46.[PubMed Abstract] -
- 21.Grebely J, Hajarizadeh B, Dore GJ. Direct-acting antiviral agents for HCV infection affecting people who inject drugs. Nat Rev Gastroenterol Hepatol. 2017;14:641-651.[PubMed Abstract] -
- 22.Grebely J, Dalgard O, Conway B, et al. Sofosbuvir and velpatasvir for hepatitis C virus infection in people with recent injection drug use (SIMPLIFY): an open-label, single-arm, phase 4, multicentre trial. Lancet Gastroenterol Hepatol. 2018;3:153-61.[PubMed Abstract] -
- 23.Dore GJ, Altice F, Litwin AH, et al. Elbasvir-Grazoprevir to Treat Hepatitis C Virus Infection in Persons Receiving Opioid Agonist Therapy: A Randomized Trial. Ann Intern Med. 2016;165:625-634.[PubMed Abstract] -
- 24.Hajarizadeh B, Cunningham EB, Reid H, Law M, Dore GJ, Grebely J. Direct-acting antiviral treatment for hepatitis C among people who use or inject drugs: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2018;3:754-67.[PubMed Abstract] -
- 25.Eckhardt B, Mateu-Gelabert P, Aponte-Melendez Y, et al. Accessible Hepatitis C Care for People Who Inject Drugs: A Randomized Clinical Trial. JAMA Intern Med. 2022;182:494-502.[PubMed Abstract] -
- 26.Grebely J, Dore GJ, Zeuzem S, et al. Efficacy and Safety of Sofosbuvir/Velpatasvir in Patients With Chronic Hepatitis C Virus Infection Receiving Opioid Substitution Therapy: Analysis of Phase 3 ASTRAL Trials. Clin Infect Dis. 2016;63:1479-1481.[PubMed Abstract] -
- 27.Grebely J, Feld JJ, Wyles D, et al. Sofosbuvir-Based Direct-Acting Antiviral Therapies for HCV in People Receiving Opioid Substitution Therapy: An Analysis of Phase 3 Studies. Open Forum Infect Dis. 2018;5:ofy001.[PubMed Abstract] -
- 28.Foster GR, Dore GJ, Wang S, et al. Glecaprevir/pibrentasvir in patients with chronic HCV and recent drug use: An integrated analysis of 7 phase III studies. Drug Alcohol Depend. 2019;194:487-94.[PubMed Abstract] -
- 29.Grebely J, Dore GJ, Alami NN, et al. Safety and efficacy of glecaprevir/pibrentasvir in patients with chronic hepatitis C genotypes 1-6 receiving opioid substitution therapy. Int J Drug Policy. 2019;66:73-9.[PubMed Abstract] -
- 30.Gonzalez-Serna A, Macias J, Corma-Gomez A, et al. High efficacy of glecaprevir/pibrentasvir for HCV-infected individuals with active drug use. J Infect. 2022;85:322-6.[PubMed Abstract] -
- 31.Grebely J, Mauss S, Brown A, et al. Efficacy and Safety of Ledipasvir/Sofosbuvir With and Without Ribavirin in Patients With Chronic HCV Genotype 1 Infection Receiving Opioid Substitution Therapy: Analysis of Phase 3 ION Trials. Clin Infect Dis. 2016;63:1405-11.[PubMed Abstract] -
- 32.Coffin PO, Santos GM, Behar E, et al. Randomized feasibility trial of directly observed versus unobserved hepatitis C treatment with ledipasvir-sofosbuvir among people who inject drugs. PLoS One. 2019;14:e0217471.[PubMed Abstract] -
- 33.Durham DP, Skrip LA, Bruce RD, et al. The Impact of Enhanced Screening and Treatment on Hepatitis C in the United States. Clin Infect Dis. 2015;62:298-304.[PubMed Abstract] -
- 34.Grebely J, Matthews GV, Lloyd AR, Dore GJ. Elimination of hepatitis C virus infection among people who inject drugs through treatment as prevention: feasibility and future requirements. Clin Infect Dis. 2013;57:1014-20.[PubMed Abstract] -
- 35.Tsui JI, Evans JL, Lum PJ, Hahn JA, Page K. Association of opioid agonist therapy with lower incidence of hepatitis C virus infection in young adult injection drug users. JAMA Intern Med. 2014;174:1974-81.[PubMed Abstract] -
- 36.McCance-Katz EF, Sullivan LE, Nallani S. Drug interactions of clinical importance among the opioids, methadone and buprenorphine, and other frequently prescribed medications: a review. Am J Addict. 2010;19:4-16.[PubMed Abstract] -
- 37.Meemken L, Hanhoff N, Tseng A, Christensen S, Gillessen A. Drug-Drug Interactions With Antiviral Agents in People Who Inject Drugs Requiring Substitution Therapy. Ann Pharmacother. 2015;49:796-807.[PubMed Abstract] -
- 38.Akiyama MJ, Norton BL, Arnsten JH, Agyemang L, Heo M, Litwin AH. Intensive Models of Hepatitis C Care for People Who Inject Drugs Receiving Opioid Agonist Therapy: A Randomized Controlled Trial. Ann Intern Med. 2019;170:594-603.[PubMed Abstract] -
- 39.Schwarz T, Horváth I, Fenz L, Schmutterer I, Rosian-Schikuta I, Mårdh O. Interventions to increase linkage to care and adherence to treatment for hepatitis C among people who inject drugs: A systematic review and practical considerations from an expert panel consultation. Int J Drug Policy. 2022;102:103588.[PubMed Abstract] -
- 40.Hajarizadeh B, Carson JM, Byrne M, et al. Incidence of hepatitis C virus infection in the prison setting: The SToP-C study. J Viral Hepat. 2024;31:21-34.[PubMed Abstract] -
- 41.Simmons B, Saleem J, Hill A, Riley RD, Cooke GS. Risk of Late Relapse or Reinfection With Hepatitis C Virus After Achieving a Sustained Virological Response: A Systematic Review and Meta-analysis. Clin Infect Dis. 2016;62:683-94.[PubMed Abstract] -
- 42.Hajarizadeh B, Cunningham EB, Valerio H, et al. Hepatitis C reinfection after successful antiviral treatment among people who inject drugs: A meta-analysis. J Hepatol. 2020;72:643-57.[PubMed Abstract] -
- 43.Munari SC, Traeger MW, Menon V, et al. Determining reinfection rates by hepatitis C testing interval among key populations: A systematic review and meta-analysis. Liver Int. 2023;43:2625-44.[PubMed Abstract] -
- 44.Hagan H, Pouget ER, Des Jarlais DC. A systematic review and meta-analysis of interventions to prevent hepatitis C virus infection in people who inject drugs. J Infect Dis. 2011;204:74-83. [PubMed Abstract] -
- 45.Cunningham EB, Jacka B, DeBeck K, et al. Methamphetamine injecting is associated with phylogenetic clustering of hepatitis C virus infection among street-involved youth in Vancouver, Canada. Drug Alcohol Depend. 2015;152:272-6.[PubMed Abstract] -
- 46.Gonzales R, Marinelli-Casey P, Shoptaw S, Ang A, Rawson RA. Hepatitis C virus infection among methamphetamine-dependent individuals in outpatient treatment. J Subst Abuse Treat. 2006;31:195-202.[PubMed Abstract] -
- 47.Hagan H, Jordan AE, Neurer J, Cleland CM. Incidence of sexually transmitted hepatitis C virus infection in HIV-positive men who have sex with men. AIDS. 2015;29:2335-45.[PubMed Abstract] -
- 48.Chou C, Yimam KK, Frederick RT, Swenson SL. A Rare Case of Icteric Acute Hepatitis C Infection Acquired Through Intranasal Methamphetamine Use. ACG Case Rep J. 2014;1:112-4.[PubMed Abstract] -
- 49.Karila L, Weinstein A, Aubin HJ, Benyamina A, Reynaud M, Batki SL. Pharmacological approaches to methamphetamine dependence: a focused review. Br J Clin Pharmacol. 2010;69:578-92.[PubMed Abstract] -
- 51.Coffin PO, Santos GM, Das M, et al. Aripiprazole for the treatment of methamphetamine dependence: a randomized, double-blind, placebo-controlled trial. Addiction. 2013;108:751-61.[PubMed Abstract] -
- 52.Colfax GN, Santos GM, Das M, et al. Mirtazapine to reduce methamphetamine use: a randomized controlled trial. Archives of general psychiatry. 2011;68:1168-75.[PubMed Abstract] -
- 53.Elkashef AM, Rawson RA, Anderson AL, et al. Bupropion for the treatment of methamphetamine dependence. Neuropsychopharmacology. 2008;33:1162-70.[PubMed Abstract] -
- 54.Shearer J, Darke S, Rodgers C, et al. A double-blind, placebo-controlled trial of modafinil (200 mg/day) for methamphetamine dependence. Addiction. 2009;104:224-33.[PubMed Abstract] -
- 55.Trivedi MH, Walker R, Ling W, et al. Bupropion and Naltrexone in Methamphetamine Use Disorder. N Engl J Med. 2021;384:140-53.[PubMed Abstract] -
- 56.Poynard T, Bedossa P, Opolon P. Lancet. Natural history of liver fibrosis progression in patients with chronic hepatitis C. The OBSVIRC, METAVIR, CLINIVIR, and DOSVIRC groups. Lancet. 1997;349:825-32.[PubMed Abstract] -
- 57.Wiley TE, McCarthy M, Breidi L, McCarthy M, Layden TJ. Impact of alcohol on the histological and clinical progression of hepatitis C infection. Hepatology. 1998;28:805-9.[PubMed Abstract] -
- 58.Innes HA, Hutchinson SJ, Barclay S, et al. Quantifying the fraction of cirrhosis attributable to alcohol among chronic HCV patients: Implications for treatment cost-effectiveness. Hepatology. 2012;57:451-60.[PubMed Abstract] -
- 59.McMahon BJ, Bruden D, Bruce MG, et al. Adverse outcomes in Alaska Natives who recovered from or have chronic hepatitis C infection. Gastroenterology. 2010;138:922-31.[PubMed Abstract] -
- 60.Bruggmann P, Dampz M, Gerlach T, Kravecz L, Falcato L. Treatment outcome in relation to alcohol consumption during hepatitis C therapy: an analysis of the Swiss Hepatitis C Cohort Study. Drug Alcohol Depend. 2010;110:167-71.[PubMed Abstract] -
- 61.Russell M, Pauly MP, Moore CD, et al. The impact of lifetime alcohol use on hepatitis C treatment outcomes in privately insured members of an integrated health care plan. Hepatology. 2012;56:1223-30.[PubMed Abstract] -
- 62.Tsui JI, Williams EC, Green PK, Berry K, Su F, Ioannou GN. Alcohol use and hepatitis C virus treatment outcomes among patients receiving direct antiviral agents. Drug Alcohol Depend. 2016;169:101-9.[PubMed Abstract] -
- 63.Garbutt JC. The state of pharmacotherapy for the treatment of alcohol dependence. Journal of substance abuse treatment. 2009;36:S15-23; quiz S24-15.[PubMed Abstract] -
- 64.Patel YA, Yao J, Proeschold-Bell RJ, Niedzwiecki D, Goacher E, Muir AJ. Reduced Alcohol Use Is Sustained in Patients Provided Alcohol-Related Counseling During Direct-Acting Antiviral Therapy for Hepatitis C. Dig Dis Sci. 2020 Sep 23. Online ahead of print.[PubMed Abstract] -
- 65.Brunet L, Moodie EE, Rollet K, et al. Marijuana smoking does not accelerate progression of liver disease in HIV-hepatitis C coinfection: A longitudinal cohort analysis. Clin Infect Dis. 2013;57:663-70.[PubMed Abstract] -
- 66.Ishida JH, Peters MG, Jin C, Louie K, Tan V, Bacchetti P, Terrault NA. Influence of cannabis use on severity of hepatitis C disease. Clin Gastroenterol Hepatol. 2008;6:69-75.[PubMed Abstract] -
- 67.Purohit V, Rapaka R, Shurtleff D. Role of cannabinoids in the development of fatty liver (steatosis). The AAPS journal. 2010;12:233-7.[PubMed Abstract] -
- 68.Nordmann S, Vilotitch A, Roux P, et al. Daily cannabis and reduced risk of steatosis in human immunodeficiency virus and hepatitis C virus-co-infected patients (ANRS CO13-HEPAVIH). J Viral Hepat. 2018;25:171-179.[PubMed Abstract] -
- 69.Sylvestre DL, Clements BJ, Malibu Y. Cannabis use improves retention and virological outcomes in patients treated for hepatitis C. Eur J Gastroenterol Hepatol. 2006;18:1057-63.[PubMed Abstract] -
Additional References
- Akiyama MJ, Agyemang L, Arnsten JH, et al. Rationale, design, and methodology of a trial evaluating three models of care for HCV treatment among injection drug users on opioid agonist therapy. BMC Infect Dis. 2018;18:74.[PubMed Abstract] -
- Butner JL, Gupta N, Fabian C, Henry S, Shi JM, Tetrault JM. Onsite treatment of HCV infection with direct acting antivirals within an opioid treatment program. J Subst Abuse Treat. 2017;75:49-53.[PubMed Abstract] -
- Cachay ER, Wyles D, Hill L, et al. The Impact of Direct-Acting Antivirals in the Hepatitis C-Sustained Viral Response in Human Immunodeficiency Virus-Infected Patients With Ongoing Barriers to Care. Open Forum Infect Dis. 2015;2:ofv168.[PubMed Abstract] -
- Elsherif O, Bannan C, Keating S, McKiernan S, Bergin C, Norris S. Outcomes from a large 10 year hepatitis C treatment programme in people who inject drugs: No effect of recent or former injecting drug use on treatment adherence or therapeutic response. PLoS One. 2017;12:e0178398.[PubMed Abstract] -
- Knight R, Roux P, Vilotitch A, et al. Significant reductions in alcohol use after hepatitis C treatment: results from the ANRS CO13-HEPAVIH cohort. Addiction. 2017;112:1669-1679.[PubMed Abstract] -
- Kosloski MP, Zhao W, Asatryan A, Kort J, Geoffroy P, Liu W. No Clinically Relevant Drug-Drug Interactions between Methadone or Buprenorphine-Naloxone and Antiviral Combination Glecaprevir and Pibrentasvir. Antimicrob Agents Chemother. 2017;61:e00958-17.[PubMed Abstract] -
- Norton BL, Fleming J, Bachhuber MA, et al. High HCV cure rates for people who use drugs treated with direct acting antiviral therapy at an urban primary care clinic. Int J Drug Policy. 2017;47:196-201.[PubMed Abstract] -
- Riley DE, Liu L, Cohen B, Robinson S, Groessl EJ, Ho SB. Characteristics and impact of methamphetamine use in patients with chronic hepatitis C. J Addict Med. 2014;8:25-32.[PubMed Abstract] -
- Substance Abuse and Mental Health Services Administration (SAMHSA). (2019). Key substance use and mental health indicators in the United States: Results from the 2018 National Survey on Drug Use and Health (HHS Publication No. PEP19-5068, NSDUH Series H-54). Rockville, MD: Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration.[SAMHSA] -
- Ye L, Peng JS, Wang X, Wang YJ, Luo GX, Ho WZ. Methamphetamine enhances Hepatitis C virus replication in human hepatocytes. J Viral Hepat. 2008;15:261-70.[PubMed Abstract] -
- Zelenev A, Li J, Mazhnaya A, Basu S, Altice FL. Hepatitis C virus treatment as prevention in an extended network of people who inject drugs in the USA: a modelling study. Lancet Infect Dis. 2018;18:215-24.[PubMed Abstract] -
Figures
 Figure 1. Number of Persons Aged 12 or Older with Past Year Illicit Drug Use, United States, 2022Source: Substance Abuse and Mental Health Services Administration (SAMHSA). (2022). Key substance use and mental health indicators in the United States: Results from the 2022 National Survey on Drug Use and Health (HHS Publication No. PEP23-07-01-006, NSDUH Series H-58). Rockville, MD: Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration.
Figure 1. Number of Persons Aged 12 or Older with Past Year Illicit Drug Use, United States, 2022Source: Substance Abuse and Mental Health Services Administration (SAMHSA). (2022). Key substance use and mental health indicators in the United States: Results from the 2022 National Survey on Drug Use and Health (HHS Publication No. PEP23-07-01-006, NSDUH Series H-58). Rockville, MD: Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration. Figure 2. Adherence with HCV Therapy in C-EDGE CO-STAR Trial*HCV treatment with elbasvir-grazoprevir in 301 PWID who were receiving opioid agonist therapy for at least 3 months prior to enrollment.Source: Dore GJ, Altice F, Litwin AH, et al. Elbasvir-Grazoprevir to Treat Hepatitis C Virus Infection in Persons Receiving Opioid Agonist Therapy: A Randomized Trial. Ann Intern Med. 2016;165:625-34.
Figure 2. Adherence with HCV Therapy in C-EDGE CO-STAR Trial*HCV treatment with elbasvir-grazoprevir in 301 PWID who were receiving opioid agonist therapy for at least 3 months prior to enrollment.Source: Dore GJ, Altice F, Litwin AH, et al. Elbasvir-Grazoprevir to Treat Hepatitis C Virus Infection in Persons Receiving Opioid Agonist Therapy: A Randomized Trial. Ann Intern Med. 2016;165:625-34. Figure 3 (Image Series). Elbasvir-Grazoprevir in PWID: C-EDGE CO-STARIn this analysis, reinfections are considered as failures.Source: Dore GJ, Altice F, Litwin AH, et al. Elbasvir-Grazoprevir to Treat Hepatitis C Virus Infection in Persons Receiving Opioid Agonist Therapy: A Randomized Trial. Ann Intern Med. 2016;165:625-634.
Figure 3 (Image Series). Elbasvir-Grazoprevir in PWID: C-EDGE CO-STARIn this analysis, reinfections are considered as failures.Source: Dore GJ, Altice F, Litwin AH, et al. Elbasvir-Grazoprevir to Treat Hepatitis C Virus Infection in Persons Receiving Opioid Agonist Therapy: A Randomized Trial. Ann Intern Med. 2016;165:625-634. Figure 3B. SVR12, by HCV Genotype (Modified Full Analysis Set^)In this analysis, reinfections are considered as responders.Source: Dore GJ, Altice F, Litwin AH, et al. Elbasvir-Grazoprevir to Treat Hepatitis C Virus Infection in Persons Receiving Opioid Agonist Therapy: A Randomized Trial. Ann Intern Med. 2016;165:625-634.
Figure 3B. SVR12, by HCV Genotype (Modified Full Analysis Set^)In this analysis, reinfections are considered as responders.Source: Dore GJ, Altice F, Litwin AH, et al. Elbasvir-Grazoprevir to Treat Hepatitis C Virus Infection in Persons Receiving Opioid Agonist Therapy: A Randomized Trial. Ann Intern Med. 2016;165:625-634. Figure 3C. SVR12 by Subgroups AnalysisDore GJ, Altice F, Litwin AH, et al. Elbasvir-Grazoprevir to Treat Source: Hepatitis C Virus Infection in Persons Receiving Opioid Agonist Therapy: A Randomized Trial. Ann Intern Med. 2016;165:625-634.
Figure 3C. SVR12 by Subgroups AnalysisDore GJ, Altice F, Litwin AH, et al. Elbasvir-Grazoprevir to Treat Source: Hepatitis C Virus Infection in Persons Receiving Opioid Agonist Therapy: A Randomized Trial. Ann Intern Med. 2016;165:625-634. Figure 4. SVR12 Response to Ledipasvir-Sofosbuvir in Persons Receiving Opioid Substitution Therapy: Phase 3 ION TrialsAbbreviations: OST = opioid substitution therapy
Figure 4. SVR12 Response to Ledipasvir-Sofosbuvir in Persons Receiving Opioid Substitution Therapy: Phase 3 ION TrialsAbbreviations: OST = opioid substitution therapy
Data from phase 3 ION trials for the subset of participants receiving OST versus those not receiving OST.Source: Grebely J, Mauss S, Brown A, et al. Efficacy and Safety of Ledipasvir/Sofosbuvir With and Without Ribavirin in Patients With Chronic HCV Genotype 1 Infection Receiving Opioid Substitution Therapy: Analysis of Phase 3 ION Trials. Clin Infect Dis. 2016;63:1405-1411. Figure 5. Impact of Alcohol Consumption on HCV Treatment ResponseIn this study, excessive alcohol consumption was defined as ≥60 g/day for men and ≥40 g/day for women.Source: Wiley TE, McCarthy M, Breidi L, McCarthy M, Layden TJ. Impact of alcohol on the histological and clinical progression of hepatitis C infection. Hepatology. 1998;28:805-9.
Figure 5. Impact of Alcohol Consumption on HCV Treatment ResponseIn this study, excessive alcohol consumption was defined as ≥60 g/day for men and ≥40 g/day for women.Source: Wiley TE, McCarthy M, Breidi L, McCarthy M, Layden TJ. Impact of alcohol on the histological and clinical progression of hepatitis C infection. Hepatology. 1998;28:805-9.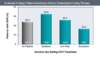 Figure 6. Impact of Alcohol Consumption on HCV Treatment ResponseInvestigators enrolled 73 patients with chronic hepatitis C (genotypes 1, 2, 3, or 4) who had ongoing alcohol consumption (or abstinence for less than 6 months) and were treated with peginterferon and ribavirin. Abstinence referred to patients off alcohol during the entire treatment period. Low-risk consumption was defined as weekly consumption of no more than 21 standard drinks for men and 14 drinks for women, and no more than 4 by drinking occasion. Excessive consumption was defined as drinking more than the limits defined for low-risk consumption on at least two occasions during the treatment period.Source: Le Lan C, Guillygomarc'h A, Danielou H, et al. A multi-disciplinary approach to treating hepatitis C with interferon and ribavirin in alcohol-dependent patients with ongoing abuse. J Hepatol. 2012;56:334-40.
Figure 6. Impact of Alcohol Consumption on HCV Treatment ResponseInvestigators enrolled 73 patients with chronic hepatitis C (genotypes 1, 2, 3, or 4) who had ongoing alcohol consumption (or abstinence for less than 6 months) and were treated with peginterferon and ribavirin. Abstinence referred to patients off alcohol during the entire treatment period. Low-risk consumption was defined as weekly consumption of no more than 21 standard drinks for men and 14 drinks for women, and no more than 4 by drinking occasion. Excessive consumption was defined as drinking more than the limits defined for low-risk consumption on at least two occasions during the treatment period.Source: Le Lan C, Guillygomarc'h A, Danielou H, et al. A multi-disciplinary approach to treating hepatitis C with interferon and ribavirin in alcohol-dependent patients with ongoing abuse. J Hepatol. 2012;56:334-40. Figure 7 (Image Series). SVR12 Rates with DAA-Based Treatment, by AUDIT-C CategoryThis study was conducted in 2014-2015Source: Tsui JI, Williams EC, Green PK, Berry K, Su F, Ioannou GN. Alcohol use and hepatitis C virus treatment outcomes among patients receiving direct antiviral agents. Drug Alcohol Depend. 2016;169:101-9.
Figure 7 (Image Series). SVR12 Rates with DAA-Based Treatment, by AUDIT-C CategoryThis study was conducted in 2014-2015Source: Tsui JI, Williams EC, Green PK, Berry K, Su F, Ioannou GN. Alcohol use and hepatitis C virus treatment outcomes among patients receiving direct antiviral agents. Drug Alcohol Depend. 2016;169:101-9. Figure 7B. Response by Cirrhosis StatusThis study was conducted in 2014-2015Source: Tsui JI, Williams EC, Green PK, Berry K, Su F, Ioannou GN. Alcohol use and hepatitis C virus treatment outcomes among patients receiving direct antiviral agents. Drug Alcohol Depend. 2016;169:101-9.
Figure 7B. Response by Cirrhosis StatusThis study was conducted in 2014-2015Source: Tsui JI, Williams EC, Green PK, Berry K, Su F, Ioannou GN. Alcohol use and hepatitis C virus treatment outcomes among patients receiving direct antiviral agents. Drug Alcohol Depend. 2016;169:101-9.Share by e-mail
Check
-On-
Learning
QuestionsThe Check-on-Learning Questions are short and topic related. They are meant to help you stay on track throughout each lesson and check your understanding of key concepts.You must be signed in to customize your interaction with these questions.
- 0%Lesson 2
Since you've received 80% or better on this quiz, you may claim continuing education credit.
You seem to have a popup blocker enabled. If you want to skip this dialog please Always allow popup windows for the online course.
Account Registration Benefits:
- Track your progress on the lessons
- Earn free CNE/CME/CE
- Earn Certificates of Completion
- Access to other free IDEA curricula
Create a free account to get started














