The treatment of hepatitis C virus (HCV) should include a pretreatment baseline evaluation, consideration of drug interactions, evaluation of treatment response after therapy, and in some populations, monitoring for safety during treatment. A typical schedule for clinic visits related to an 8- or 12-week treatment course of direct-acting antiviral (DAA) therapy would consist of a baseline visit just prior to starting therapy, a follow-up visit at week 4 of therapy, an end-of-treatment visit, and a post-treatment visit 12 weeks after completing therapy. Depending on the individual’s specific circumstances, follow-up visits may be done via phone or telehealth. For longer courses of treatment, such as a 16-week treatment course, most clinicians would add one or more on-treatment visits. Any person receiving treatment who develops a significant adverse event or complication related to DAAs should be seen and evaluated in additional visits, as needed. In addition, individuals with cirrhosis or other complicating conditions may require more frequent follow-up. This topic review addresses the recommendations for monitoring the safety and efficacy during treatment, as well as considerations for monitoring persons after treatment.
- Module 5 Overview
Treatment of Hepatitis C Infection - 0%Lesson 1
Simplified HCV Treatment for All HCV GenotypesActivities- 0%Lesson 2
Retreatment of Patients with Prior HCV Treatment ExperienceActivities- 0%Lesson 3
Monitoring During and After HCV TreatmentLesson 3. Monitoring During and After HCV Treatment
PDF ShareLast Updated: October 5th, 2023Authors:Maria A. Corcorran, MD, MPH,Maria A. Corcorran, MD, MPH
Associate Editor
Assistant Professor
Division of Allergy & Infectious Diseases
University of WashingtonDisclosures: NoneH. Nina Kim, MD, MScH. Nina Kim, MD, MSc
Associate Editor
Professor of Medicine
Division of Allergy & Infectious Diseases
University of WashingtonDisclosures: NoneReviewer:David H. Spach, MDDavid H. Spach, MD
Editor-in-Chief
Professor of Medicine
Division of Allergy & Infectious Diseases
University of WashingtonDisclosures: NoneLearning Objective Performance Indicators
- List the recommended monitoring for treatment efficacy and safety in adults receiving HCV therapy
- Discuss monitoring for hepatitis B virus reactivation during HCV therapy
- Summarize the approach to persons with elevated alanine aminotransferase levels (ALT) during therapy
- Define sustained virologic response
- Describe appropriate monitoring for patients after HCV treatment
Table of ContentsBackground
Monitoring for Treatment Efficacy
Recommended Method for Monitoring of Treatment Efficacy
The standard approach to monitoring for treatment efficacy consists of measuring quantitative HCV RNA levels. Monitoring requires use of a highly sensitive quantitative HCV RNA assay, typically with a lower limit of quantification in the range of 12 to 25 IU/mL.[1] Three commercially available HCV RNA assays are widely used in the United States: Roche COBAS TaqMan Version 1.0, Roche COBAS TaqMan Version 2.0, and the Abbott RealTime HCV (ART) assay.[2,3,4,5,6] The following definitions related to HCV RNA assay results are used in clinical practice and in research studies (Figure 1):[4]
- Lower Limit of Quantification (LLOQ): This is the lowest HCV RNA concentration that is within the validated range the assay can accurately quantify. If the HCV RNA level is not quantifiable, the result is either HCV RNA detected but below the LLOQ or HCV RNA not detected. Note that the lower limit of quantification is not the same as the lower limit of detection.
- Limit of Detection (LOD): This value is the concentration of HCV RNA detectable at a rate of at least 95%. The ability of the assay to detect HCV RNA gradually decreases as the actual amount of HCV RNA in the sample approaches 0 IU/mL. The result below the limit of detection is referred to as undetectable.
- Target (or Viral RNA) Detected (TD): The HCV RNA is detected.
- Target (or Viral RNA) Not Detected (TND): The HCV RNA is not detected.
Recommended Schedule for HCV RNA Monitoring
For individuals receiving HCV therapy, the AASLD-IDSA HCV Guidance recommends obtaining a quantitative HCV RNA level at baseline and at 12 weeks after completing therapy, regardless of the treatment duration. Typical treatment durations are 8 weeks (Figure 2) and 12 weeks (Figure 3).[7]
On-Treatment HCV RNA Monitoring
For persons on DAA-based therapy, routine HCV RNA testing at week 4 is no longer recommended.[7] Phase 3 trials with DAAs have demonstrated that nearly all patients without cirrhosis had an HCV RNA level at week 4 that was undetectable (or less than the LLOQ).[8] Observational data from the Veterans Administration suggest that detectable HCV RNA (15 IU/mL or greater) at week 4 may be associated with lower odds of sustained virologic response (SVR) at 12 weeks after completion of therapy.[9] Nevertheless, SVR has been well documented among individuals who have a higher-than-expected or detectable HCV RNA level at week 4 of treatment.[10] The AASLD-IDSA HCV Guidance now advises against routine testing for HCV RNA level during treatment unless (1) alanine aminotransferase (ALT) fails to decline from baseline (in persons where hepatic function monitoring is indicated and ongoing) or (2) there is concern regarding patient adherence with DAA therapy. The panel also notes there are no data to support HCV treatment cessation or modification based on a detectable level at week 4 of treatment. It is also not clear that a detectable HCV RNA anytime during therapy reflects nonadherence.[7]
On-Treatment Persistent Low-Level Viremia
Furthermore, the significance of on-treatment persistent low-level viremia (that does not increase) is not known. There is no clear indication this represents a lack of adherence or likelihood of virologic relapse, and routine on-treatment monitoring of HCV RNA levels is not indicated. Indeed, recent data involving individuals receiving sofosbuvir-containing DAA therapy has shown that low-level quantifiable HCV RNA levels at week 4 were not clinically useful in predicting SVR12; these findings contrast sharply with prior studies using interferon-based regimens.[8] The AASLD-IDSA HCV Guidance does not provide specific guidance regarding stopping or extending therapy in the setting of stable low-level viremia.[7]
Determining Sustained Virologic Response
The recommended testing to determine whether the patient has achieved an SVR is a quantitative HCV RNA level 12 weeks after completing therapy (Figure 4).[11,12] An undetectable HCV RNA level 12 weeks after completing therapy is referred to as SVR12, which translates into a long-term cure of HCV infection.[11,13,14] Some experts will obtain an HCV RNA level 24 weeks after completing treatment in selected patients, such as those with cirrhosis, in order to confirm lack of virologic relapse. Research studies have utilized HCV RNA levels 4 weeks after completing therapy (SVR4), but while the SVR4 can be associated with SVR12, it is not considered as robust a marker for durable treatment response as SVR12.[15]
Monitoring for Safety at Baseline and During Treatment
Baseline Safety Laboratory Studies
The AASLD-IDSA HCV Guidance recommends obtaining the following baseline studies within 6 months prior to starting treatment:[7]
- Complete blood count (CBC)
- Hepatic function panel: albumin, total bilirubin, direct bilirubin, alanine aminotransferase (ALT), aspartate aminotransferase (AST), and alkaline phosphatase
- International normalized ratio (INR)
- Serum creatinine level and calculated glomerular filtration rate
- A serum pregnancy test for all women of childbearing age prior to treatment, especially if the HCV treatment regimen includes ribavirin, which is a known teratogen.
- Testing for HCV RNA can be completed anytime prior to starting DAA therapy, and an HCV genotype and subtype should be obtained if the planned treatment regimen is not a pangenotypic regimen.
- Hepatitis A IgG to assess for hepatitis A immunity (or need for vaccination)
- Assess for coinfection with hepatitis B virus (HBV) with an hepatitis B surface antigen (HBsAg), hepatitis B core antibody (anti-HBc), and hepatitis B surface antibody (anti-HBs)
- Assess for coinfection with HIV with an HIV-1/2 antigen-antibody immunoassay
Safety Issues with Ribavirin
The use of ribavirin for the treatment of HCV has markedly declined in recent years, but ribavirin may occasionally be indicated as an adjunct to DAA therapy in treatment-experienced individuals or those with decompensated cirrhosis. Ribavirin can cause severe hemolytic anemia, especially at higher doses. For persons taking ribavirin, regular monitoring of hemoglobin is recommended. Ribavirin is a teratogenic drug in rodents and may cause birth defects and fetal harm when administered to women who are pregnant. It is therefore contraindicated in pregnant women and in men whose female partners are pregnant. In addition, extreme care must be taken to prevent pregnancy in females taking ribavirin and in female partners of male patients taking ribavirin. Accordingly, ribavirin should not be started unless there is a documented report of a negative pregnancy test immediately prior to the planned initiation of ribavirin. Women taking ribavirin (and women who have a male partner taking ribavirin) should be instructed to use at least two forms of effective contraception during treatment that includes ribavirin and for 6 months after treatment has been stopped. Women receiving ribavirin (and women who have a male partner taking ribavirin) should have monthly pregnancy tests during ribavirin treatment and for 6 months after treatment has been completed.[7]
On-Treatment Laboratory Monitoring
The AASLD-IDSA HCV Guidance does not recommend routine on-treatment laboratory monitoring. In select groups, however, the following laboratory studies are recommended while on treatment:[7]
- Obtain hepatic function panel (albumin, total bilirubin, direct bilirubin, ALT, AST, and alkaline phosphatase) and HBV DNA every 4 weeks for persons with chronic hepatitis B who are not taking HBV therapy
- Monitor CBC if the person is taking ribavirin, typically beginning 2 weeks after initiation of ribavirin and continuing every 4 weeks (or more frequently as needed)
- Monitor for hypoglycemia for individuals taking diabetes medications
- Monitoring of INR in persons taking warfarin
- Persons receiving elbasvir-grazoprevir should have a hepatic function panel obtained after 8 weeks of treatment. This recommendation stems from the clinical trials of elbasvir-grazoprevir, in which 1% of participants experienced ALT elevations greater than 5 times the upper limit of normal. Patients receiving a 16-week course of elbasvir-grazoprevir should undergo additional hepatic function laboratory testing at 12 weeks.
Management of Abnormal ALT During Therapy
Clinically significant hepatotoxicity has been reported with DAA therapy, specifically NS3 protease inhibitors, particularly for patients with advanced liver disease. The AASLD-IDSA HCV Guidance advises against the use of NS3 protease inhibitors in patients with decompensated cirrhosis (Child-Turcotte-Pugh score of 7 or greater). Elevations in ALT to greater than 5 times the upper limit of normal occurs in up to 1% of all persons taking elbasvir-grazoprevir; therefore, hepatic panel monitoring for persons taking elbasvir-grazoprevir has been recommended by the manufacturer at week 8 and as clinically indicated during therapy (some experts recommend monitoring of ALT every 4 weeks while on elbasvir-grazoprevir). Due to the increased risk of hepatotoxicity, coadministration of ethinyl estradiol with glecaprevir-pibrentasvir is not recommended. For individuals who have on-treatment increases in ALT levels at week 4, the AASLD-IDSA HCV Guidance provides the following recommendations based on the severity of the ALT elevation and whether symptoms are present.
- A 10-fold or Greater Increase in ALT Levels: If a patient receiving treatment for HCV develops a 10-fold or greater increase in ALT levels, regardless of the presence of clinical symptoms, they should immediately stop HCV therapy and undergo close clinical and laboratory monitoring for liver toxicity.
- Clinical Symptoms and Increase in ALT Levels of Less than 10-Fold: If a patient receiving treatment for HCV has elevated ALT levels that are less than a 10-fold increase, they should immediately stop therapy if they have either of the following: (1) symptoms suggestive of acute hepatitis (weakness, nausea, vomiting, or jaundice), or (2) a significant increase in other hepatic function panel labs (bilirubin, alkaline phosphatase, or international normalized ratio). After stopping therapy, the individual should have close clinical and laboratory monitoring for liver toxicity. The AASLD-IDSA HCV Guidance does not specify what degree of change in bilirubin, alkaline phosphatase, or international normalized ratio should be considered as significant. Most experts would use clinical judgment with this recommendation.
- Asymptomatic and Increases in ALT Levels Less than 10-Fold: Patients with an increase in ALT levels less than 10-fold, but without symptoms suggestive of acute hepatitis, should have close monitoring and repeat ALT levels checked at 2-week intervals. If the ALT levels remain consistently elevated, discontinuation of therapy should be considered. In addition to the AASLD-IDSA HCV Guidance, decisions regarding discontinuation of therapy should take into account the degree of ALT elevation, the trend in ALT levels, and the presence or absence of underlying cirrhosis or symptoms of hepatic injury.
Hepatitis B Reactivation Associated with HCV DAA Therapy
Background
Hepatitis B virus (HBV) reactivation associated with severe hepatitis flare is a potentially severe adverse event associated with HCV DAA therapy.[16] Previous reports have described HBV reactivation after interferon-based therapy, but in these prior cases, clinically significant hepatitis was rare. Chronic HCV has been known to suppress HBV replication in persons with confection, and a reciprocal interaction between these viruses has long been postulated.[17] The elimination of HCV can result in a potential loss of immunologic control of HBV infection and result in HBV reactivation.
Food and Drug Administration Warning and Adverse Event Reporting Data
The U.S. Food and Drug Administration (FDA) issued a drug safety warning on October 4, 2016, in which they identified 24 cases of confirmed reactivation of HBV infection in persons receiving DAA medications for treatment of HCV.[18] The FDA warning was based on a number of cases reported to the FDA and from published literature.[19,20,21,22,23,24] The FDA later published findings that summarized a total of 29 patients (5 from the United States) with confirmed HBV reactivation during DAA therapy; their summary was based on published reports as well as cases detected via their Adverse Event Reporting System database between November 2013 and October 2016.[25] Key findings from this report included:
- Unexpected ALT and AST elevations after starting DAAs were a common feature in all these cases, occurring typically 4 to 8 weeks (mean 53 days) from treatment initiation and, in approximately one-third of the cases, the initial suspected diagnosis was an adverse drug reaction caused by DAA hepatotoxicity.
- The DAA regimens used in the treatment of these patients were heterogeneous and included sofosbuvir, simeprevir, daclatasvir with asunaprevir (investigational), and ledipasvir-sofosbuvir. Simeprevir and daclatasvir are no longer manufactured in the United States. Hospitalization occurred in at least 6 patients.
- Severe clinical decompensation occurred in 3 cases, resulting in 2 deaths and 1 liver transplantation.
- Among the 29 cases of HBV reactivation, 13 (45%) occurred in chronic HBV carriers with positive hepatitis B surface antigen (HBsAg). There were notably some patients who had absent HBsAg and anti-HBs and an isolated anti-HB core profile among these cases. In many of the cases, some serologic data were missing.
- Although the anti-HB core and anti-HB surface antibody status were not known in most cases, none of the patients with HBV reactivation had positive anti-HBs surface antibody (Figure 5).
- Antiviral treatment of HBV was initiated in 15 patients, and in most cases resulted in improvement of the liver enzymes as well as symptoms of fatigue and malaise.
Additional Data on HBV Reactivation During Therapy
A prospective study from China evaluated 327 patients in an HBV-endemic area who were scheduled to receive DAA therapy for chronic HCV infection.[26] At baseline, 10 were HBsAg-positive. After starting DAA therapy for HCV, 30% (3 of 10) of the HBsAg-positive patients experienced a hepatitis flare with HBV reactivation versus none of the 317 who were HBsAg-negative, including 124 with occult HBV infection.[26] For the three patients who developed a hepatitis flare, the median time for onset of the flare was 8 weeks. Two retrospective studies, both based on national data from the U.S. Department of Veterans Affairs, suggested that HBV reactivation is a rare event and most likely to occur in HBsAg-positive patients.[27,28]
AASLD-IDSA HCV Guidance Related to HBV Reactivation
The AASLD-IDSA HCV Guidance provides specific recommendations that address the risk of HBV reactivation following initiation of DAA treatment for HCV as summarized in the following list:[7]
- All persons about to initiate HCV DAA therapy should undergo assessment for HBV coinfection with HBsAg, anti-HB core, and anti-HBs.
- If the HBV serologic testing indicates the patient is susceptible to HBV infection, they should receive the hepatitis B vaccine series.
- For individuals who test positive for HBsAg, follow-up HBV DNA testing should be performed. If the HBsAg-positive individual is not receiving HBV suppressive therapy, treatment for HBV should be given if treatment criteria are met based on HBV DNA and ALT levels.[29]
- If treatment of HBV is indicated, it should occur before, or if necessary, at the same time as starting HCV DAA therapy. Many experts wait one month to initiate DAA therapy after initiating antivirals for HBV.
- For patients who are HBsAg-positive and do not meet the criteria for imitating HBV antiviral therapy, options include either (1) prophylactic HBV therapy, or (2) monitoring HBV DNA levels at regular intervals (but generally not more often than every 4 weeks) during HCV DAA therapy.
- If an individual who did not meet the criteria for HBV therapy is undergoing HBV DNA monitoring during HCV DAA therapy and has a 10-fold increase in HBV DNA levels, or a single HBV DNA level greater than 1,000 IU/mL, then treatment for HBV should be started.
- There are limited data on the outcomes of persons with isolated anti-HBc, or those who are positive both for anti-HBc and anti-HBs that would inform recommendations. It is possible that persons with isolated anti-HBc could reactivate HBV during HCV DAA therapy, though this event appears to rarely occur in such patients. Some experts recommend repeating hepatic function testing in patients who are anti-HBc positive (regardless of anti-HBs status) at monthly intervals during DAA therapy.
Authors’ Recommendations
We recommend obtaining baseline HBsAg, anti-HB core, and anti-HBs prior to starting HCV DAA therapy to evaluate for risk of HBV reactivation during DAA therapy for HCV. These baseline laboratory studies are not necessary for an individual whose HBV status (immune or infected) is already known from prior serologic testing or immunization. Repeat testing would be indicated in an individual with negative prior testing or no available baseline testing. Individuals with a positive baseline HBsAg should have a baseline HBV DNA level ordered. Based on results from this baseline evaluation, we recommend the following:
- Individuals with a positive HBsAg should receive therapy for HBV to prevent reactivation during HCV DAA therapy. Our recommendation is based on the significant risk of HBV reactivation in persons with positive HBsAg and the potential severity of the hepatitis flares associated with this resurgence.[25,26] The treatment for HBV should begin prior to or concomitant with initiation of HCV DAA therapy, preferably 2 to 4 weeks in advance of starting HCV therapy. Assuming the patient has not previously received HBV therapy, we recommend treatment of HBV with one of the following three regimens: entecavir 0.5 mg orally once daily, tenofovir disoproxil fumarate 300 mg orally once daily, or tenofovir alafenamide 25 mg orally once daily. The treatment of HBV should continue for at least 3 months following completion of HCV DAA therapy, with possible discontinuation of HBV treatment if the individual did not have an indication for chronic HBV therapy at baseline; preferably, this decision should be made with expert consultation.
- Individuals who are anti-HB core positive, but HBsAg and anti-HBs negative, may have a risk of HBV reactivation during HCV DAA therapy, but the risk is significantly lower than in persons who are HBsAg positive.[25,26,30] For patients with an isolated anti-HB core positive test, we recommend monitoring ALT and AST levels every 4 weeks during HCV DAA therapy; if these levels increase greater than two-fold from baseline, then obtain a quantitative HBV DNA level. Consider initiating HBV therapy (with a regimen outlined above) if the HBV DNA is detectable.
- For individuals who are anti-HBs positive, HBsAg negative, and anti-HB core positive, we do not recommend HBV DNA testing or treatment to prevent HBV reactivation. We consider ALT and AST monitoring as optional in these patients.
- We do not recommend monitoring for HBV reactivation in persons who have a positive anti-HBs and negative anti-HB core (i.e. individuals who received hepatitis B vaccination but have never been exposed to HBV infection naturally).
Monitoring After Receiving HCV Therapy
Approach to Monitoring After Receiving HCV Therapy
The approach to monitoring individuals following the completion of a course of HCV therapy depends entirely on the person’s response to therapy. Two main scenarios exist: (1) the person achieved an SVR12, or (2) the person did not achieve an SVR, including persons who completed therapy and persons who received an inadequate treatment course because of adherence problems, intolerance, or laboratory toxicity necessitating premature discontinuation of the treatment regimen.
Monitoring Persons Who Achieve SVR
Individuals who have an undetectable HCV RNA at week 12 after completing HCV therapy (or later than 12 weeks) are considered to have achieved a virologic cure, and this is associated with long-term reduced liver-related morbidity and mortality.[11,31,32] In a review of 44 studies involving more than 4,228 patients who achieved an SVR with an interferon-based regimen, 97% of patients maintained the SVR during the long-term follow-up period.[33] Some experts will obtain an HCV RNA level 24 weeks after completing treatment in selected individuals, such as those with cirrhosis. In a review by Manns, more than 99.2% of 1,002 study participants who achieved an SVR12 with interferon- or peginterferon-based therapy maintained undetectable HCV RNA levels for 5 years.[13] Available long-term data for the durability of treatment response with all-oral DAA therapy suggest SVR12 responses translate into sustained HCV clearance.[34] All patients who achieve an SVR should clearly understand they are not immune to HCV and can become reinfected with HCV.[14,34,35,36] The AASLD-IDSA HCV Guidance stratifies the follow-up for persons who achieve an SVR based on the degree of hepatic fibrosis and the risk of HCV reinfection.[7]
- Individuals without Cirrhosis: These individuals do not need special monitoring or follow-up specifically for hepatitis C or liver care. This recommendation is based on data that show persons who achieve SVR following hepatitis C treatment generally do not have further progression of HCV-related liver fibrosis.
- Individuals with Cirrhosis: Although fibrosis may improve in these individuals, they are considered to have a persistent risk of developing hepatocellular carcinoma (HCC). Accordingly, these individuals should have continued surveillance for HCC with an abdominal ultrasound (with or without alpha-fetoprotein) every 6 months.[37] In addition, persons with cirrhosis should have a baseline upper endoscopy to screen for varices, unless this has previously been done. Individuals identified with varices should receive appropriate management and follow-up.[38]
- Individuals with Persistently Abnormal Liver Tests: Any person who has achieved an SVR12, but who has persistently elevated ALT levels should undergo evaluation for possible other causes of liver disease, such as alcohol use, iron overload, or fatty liver disease.
- Persons with Ongoing Risk of HCV Reinfection: All persons with ongoing risk for acquiring HCV should have periodic assessment for HCV reinfection and counseling on prevention of reinfection. At least annual HCV RNA screening is recommended for persons who inject drugs, for men with HIV who have condomless sex with men, and for men who are taking HIV preexposure prophylaxis (PrEP) and having sex with men, given the significant risk of HCV reinfection in these individuals. Obtaining an HCV antibody does not provide useful information in persons with known prior HCV infection since they are likely to remain antibody-positive. Thus, HCV screening for reinfection should consist of a quantitative HCV RNA level. In addition, for these individuals, any elevation in hepatic aminotransferase levels should prompt evaluation for reinfection with a quantitative HCV RNA level.
Monitoring of Persons Who Do Not Achieve SVR
The AASLD-IDSA HCV Guidance recommends the following for individuals who did not achieve an SVR with HCV therapy:[7]
- All Individuals: For all individuals who did not achieve an SVR and are awaiting or deferring retreatment, follow-up laboratory testing should occur every 6-12 months with a hepatic function panel, complete blood count, and international normalized ratio. It is important these patients receive counseling for alcohol abstinence and avoidance of hepatotoxic medications (see NIH Liver Tox).
- Persons with Cirrhosis: These individuals should have surveillance for hepatocellular carcinoma with abdominal ultrasound, with or without serum alpha-fetoprotein, every 6 months.[37] In addition, individuals with cirrhosis (F4 fibrosis) should have a baseline upper endoscopy to screen for varices unless this has previously been done.[38] Persons identified with varices should receive appropriate management and follow-up.[38]
Summary Points
- All persons undergoing treatment for hepatitis C need a laboratory evaluation before and 12 weeks after therapy. In select instances, laboratory monitoring may also be indicated while on hepatitis C treatment.
- Quantitative HCV RNA is the preferred test for monitoring response to treatment, ideally with a lower limit of quantification in the range of 12 to 25 IU/mL. Patients should have a quantitative HCV RNA level obtained at baseline prior to starting therapy and 12 weeks after completion of treatment.
- An undetectable HCV RNA at 12 weeks after treatment is considered a sustained virologic response and effectively a cure for nearly all patients.
- Safety laboratory studies should be obtained at baseline. Further safety laboratory monitoring (e.g., monthly) may be required in specific circumstances and/or in the case of abnormal results.
- All persons of childbearing potential should be advised of the teratogenic potential of ribavirin. If its use is necessary, patients should be instructed to use at least two forms of effective contraception during any treatment that includes ribavirin and for 6 months after treatment has been stopped. A pregnancy test is required at baseline, monthly during ribavirin treatment, and for 6 months after completing ribavirin treatment. These same precautions and recommendations exist for women with male partners taking ribavirin.
- All persons with a 10-fold or greater increase in ALT levels while on DAAs should have therapy promptly discontinued with close follow-up. In most circumstances, individuals with symptoms suggestive of acute hepatic injury and increases in ALT that are less than 10-fold should discontinue therapy.
- Hepatitis B virus reactivation associated with a severe hepatitis flare has been increasingly recognized as a potential adverse event associated with HCV DAA therapy. The highest risk has been observed with HBsAg-positive patients, but HBV reactivation has been reported in persons with isolated anti-HBc.
- Monitoring of patients after treatment depends on whether the patient achieves an SVR12 and whether they have cirrhosis.
- Patients with an SVR12 should receive education and counseling on the risk of becoming reinfected with HCV. At least annual quantitative HCV RNA testing for reinfection is recommended for persons who inject drugs, men with HIV who have condomless sex with other men, and men who are on HIV PrEP and are having sex with men.
- Individuals with cirrhosis require long-term surveillance for HCC, regardless of whether they achieve an SVR12.
- Persons who do not achieve an SVR12 following hepatitis C treatment should continue to have regular follow-up and periodic reassessment for retreatment.
Citations
- 1.Harrington PR, Deming DJ, Komatsu TE, Naeger LK. Hepatitis C virus RNA levels during interferon-free combination direct-acting antiviral treatment in registrational trials. Clin Infect Dis. 2015;61:666-7.[PubMed Abstract] -
- 2.Cloherty G, Cohen D, Sarrazin C, et al. HCV RNA assay sensitivity impacts the management of patients treated with direct-acting antivirals. Antivir Ther. 2014;20:177-83.[PubMed Abstract] -
- 3.Chevaliez S, Bouvier-Alias M, Brillet R, Pawlotsky JM. Overestimation and underestimation of hepatitis C virus RNA levels in a widely used real-time polymerase chain reaction-based method. Hepatology. 2007;46:22-31.[PubMed Abstract] -
- 4.Cobb B, Pockros PJ, Vilchez RA, Vierling JM. HCV RNA viral load assessments in the era of direct-acting antivirals. Am J Gastroenterol. 2013;108:471-5.[PubMed Abstract] -
- 5.Vermehren J, Kau A, Görtner BC, Göbel R, Zeuzem S, Sarrazin C. Differences between two real-time PCR-based hepatitis C virus (HCV) assays (RealTime HCV and Cobas AmpliPrep/Cobas TaqMan) and one signal amplification assay (Versant HCV RNA 3.0) for RNA detection and quantification. J Clin Microbiol. 2008;46:3880-91. [PubMed Abstract] -
- 6.Strassl R, Rutter K, Stättermayer AF, et al. Real-Time PCR Assays for the Quantification of HCV RNA: Concordance, Discrepancies and Implications for Response Guided Therapy. PLoS One. 2015;10:e0135963.[PubMed Abstract] -
- 7.AASLD-IDSA. HCV Guidance: Recommendations for testing, management, and treating hepatitis C. Monitoring patients who are starting HCV treatment, are on treatment, or have completed therapy.
- 8.Sidharthan S, Kohli A, Sims Z, et al. Utility of hepatitis C viral load monitoring on direct-acting antiviral therapy. Clin Infect Dis. 2015;60:1743-51.[PubMed Abstract] -
- 9.Backus LI, Belperio PS, Shahoumian TA, Loomis TP, Mole LA. Real-world effectiveness of ledipasvir/sofosbuvir in 4,365 treatment-naive, genotype 1 hepatitis C-infected patients. Hepatology. 2016;64:405-14.[PubMed Abstract] -
- 10.Childs-Kean LM, Hong J. Detectable Viremia at the End of Treatment With Direct-Acting Antivirals Can Be Associated With Subsequent Clinical Cure in Patients With Chronic Hepatitis C: A Case Series. Gastroenterology. 2017;153:1165-1166.[PubMed Abstract] -
- 11.Morisco F, Granata R, Stroffolini T, et al. Sustained virological response: a milestone in the treatment of chronic hepatitis C. World J Gastroenterol. 2013;19:2793-8.[PubMed Abstract] -
- 12.Pearlman BL, Traub N. Sustained virologic response to antiviral therapy for chronic hepatitis C virus infection: a cure and so much more. Clin Infect Dis. 2011;52:889-900.[PubMed Abstract] -
- 13.Manns MP, Pockros PJ, Norkrans G, et al. Long-term clearance of hepatitis C virus following interferon α-2b or peginterferon α-2b, alone or in combination with ribavirin. J Viral Hepat. 2013;20:524-9.[PubMed Abstract] -
- 14.Simmons B, Saleem J, Hill A, Riley RD, Cooke GS. Risk of Late Relapse or Reinfection With Hepatitis C Virus After Achieving a Sustained Virological Response: A Systematic Review and Meta-analysis. Clin Infect Dis. 2016;62:683-94.[PubMed Abstract] -
- 15.Yoshida EM, Sulkowski MS, Gane EJ, et al. Concordance of sustained virological response 4, 12, and 24 weeks post-treatment with sofosbuvir-containing regimens for hepatitis C virus. Hepatology. 2015;61:41-5.[PubMed Abstract] -
- 16.Pisaturo M, Macera M, Alessio L, Calò F, Coppola N. Hepatitis B Virus (HBV) Reactivation Following Pharmacological Eradication of Hepatitis C Virus (HCV). Viruses. 2019;11:850.[PubMed Abstract] -
- 17.Zhang K, Lai X, Song J, et al. A novel cell culture model reveals the viral interference during hepatitis B and C virus coinfection. Antiviral Res. 2021;189:105061.[PubMed Abstract] -
- 18.U.S. Food and Drug Administration. FDA Drug Safety Communication: FDA warns about the risk of hepatitis B reactivating in some patients treated with direct-acting antivirals for hepatitis C.
- 19.Ou P, Fang Z, Chen J. Hepatitis B reactivation in a chronic hepatitis C patient treated with ledipasvir and sofosbuvir: A case report. Clin Res Hepatol Gastroenterol. 2017;41:e17-e18.[PubMed Abstract] -
- 20.Takayama H, Sato T, Ikeda F, Fujiki S. Reactivation of hepatitis B virus during interferon-free therapy with daclatasvir and asunaprevir in patient with hepatitis B virus/hepatitis C virus co-infection. Hepatol Res. 2016;46:489-91.[PubMed Abstract] -
- 21.Hayashi K, Ishigami M, Ishizu Y, et al. A case of acute hepatitis B in a chronic hepatitis C patient after daclatasvir and asunaprevir combination therapy: hepatitis B virus reactivation or acute self-limited hepatitis? Clin J Gastroenterol. 2016;9:252-6.[PubMed Abstract] -
- 22.Ende AR, Kim NH, Yeh MM, Harper J, Landis CS. Fulminant hepatitis B reactivation leading to liver transplantation in a patient with chronic hepatitis C treated with simeprevir and sofosbuvir: a case report. J Med Case Rep. 2015;9:164.[PubMed Abstract] -
- 23.De Monte A, Courjon J, Anty R, et al. Direct-acting antiviral treatment in adults infected with hepatitis C virus: Reactivation of hepatitis B virus coinfection as a further challenge. J Clin Virol. 2016;78:27-30.[PubMed Abstract] -
- 24.Collins JM, Raphael KL, Terry C, et al. Hepatitis B Virus Reactivation During Successful Treatment of Hepatitis C Virus With Sofosbuvir and Simeprevir. Clin Infect Dis. 2015;61:1304-6.[PubMed Abstract] -
- 25.Bersoff-Matcha SJ, Cao K, Jason M, et al. Hepatitis B Virus Reactivation Associated With Direct-Acting Antiviral Therapy for Chronic Hepatitis C Virus: A Review of Cases Reported to the U.S. Food and Drug Administration Adverse Event Reporting System. Ann Intern Med. 2017;166:792-8.[PubMed Abstract] -
- 26.Wang C, Ji D, Chen J, et al. Hepatitis due to Reactivation of Hepatitis B Virus in Endemic Areas Among Patients With Hepatitis C Treated With Direct-acting Antiviral Agents. Clin Gastroenterol Hepatol. 2017;15:132-6.[PubMed Abstract] -
- 27.Belperio PS, Shahoumian TA, Mole LA, Backus LI. Evaluation of hepatitis B reactivation among 62,920 veterans treated with oral hepatitis C antivirals. Hepatology. 2017;66:27-36.[PubMed Abstract] -
- 28.Serper M, Forde KA, Kaplan DE. Rare clinically significant hepatic events and hepatitis B reactivation occur more frequently following rather than during direct-acting antiviral therapy for chronic hepatitis C: Data from a national US cohort. J Viral Hepat. 2018;25:187-197.[PubMed Abstract] -
- 29.Terrault NA, Bzowej NH, Chang KM, Hwang JP, Jonas MM, Murad MH. AASLD guidelines for treatment of chronic hepatitis B. Hepatology. 2016;63:261-83.[PubMed Abstract] -
- 30.Sulkowski MS, Chuang WL, Kao JH, et al. No Evidence of Reactivation of Hepatitis B Virus Among Patients Treated With Ledipasvir-Sofosbuvir for Hepatitis C Virus Infection. Clin Infect Dis. 2016;63:1202-1204.[PubMed Abstract] -
- 31.Morgan RL, Baack B, Smith BD, Yartel A, Pitasi M, Falck-Ytter Y. Eradication of hepatitis C virus infection and the development of hepatocellular carcinoma: a meta-analysis of observational studies. Ann Intern Med. 2013;158:329-37.[PubMed Abstract] -
- 32.Singal AG, Volk ML, Jensen D, Di Bisceglie AM, Schoenfeld PS. A sustained viral response is associated with reduced liver-related morbidity and mortality in patients with hepatitis C virus. Clin Gastroenterol Hepatol. 2010;8:280-8.[PubMed Abstract] -
- 33.Welker MW, Zeuzem S. Occult hepatitis C: how convincing are the current data? Hepatology. 2009;49:665-75.[PubMed Abstract] -
- 34.Sarrazin C, Isakov V, Svarovskaia ES, et al. Late Relapse Versus Hepatitis C Virus Reinfection in Patients With Sustained Virologic Response After Sofosbuvir-Based Therapies. Clin Infect Dis. 2017;64:44-52.[PubMed Abstract] -
- 35.Ingiliz P, Martin TC, Rodger A, et al. HCV reinfection incidence and spontaneous clearance rates in HIV-positive men who have sex with men in Western Europe. J Hepatol. 2017;66:282-287.[PubMed Abstract] -
- 36.Martin TC, Martin NK, Hickman M, et al. Hepatitis C virus reinfection incidence and treatment outcome among HIV-positive MSM. AIDS. 2013;27:2551-7.[PubMed Abstract] -
- 37.Heimbach JK, Kulik LM, Finn RS, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67:358-80.[PubMed Abstract] -
- 38.Garcia-Tsao G, Abraldes JG, Berzigotti A, Bosch J. Portal hypertensive bleeding in cirrhosis: Risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the study of liver diseases. Hepatology. 2017;65:310-335.[PubMed Abstract] -
Additional References
- European Association for the Study of the Liver. EASL recommendations on treatment of hepatitis C: Final update of the series☆. J Hepatol. 2020:S0168-8278(20)30548-1.[EASL] -
- Steinebrunner N, Sprinzl MF, Zimmermann T, et al. Early virological response may predict treatment response in sofosbuvir-based combination therapy of chronic hepatitis c in a multi-center "real-life" cohort. BMC Gastroenterol. 2015;15:97.[PubMed Abstract] -
Figures
 Figure 1. HCV RNA Assay ReportsThis graphic illustrates sample cutoffs for lower limit of quantification (LLOQ) and limit of detection (LOD) for HCV RNA values. In this example, the HCV RNA assay has an LLOQ of 25 IU/mL and an LOD of 10 IU/mL.
Figure 1. HCV RNA Assay ReportsThis graphic illustrates sample cutoffs for lower limit of quantification (LLOQ) and limit of detection (LOD) for HCV RNA values. In this example, the HCV RNA assay has an LLOQ of 25 IU/mL and an LOD of 10 IU/mL.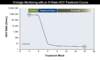 Figure 2. Monitoring of Quantitative HCV RNA Levels in Persons Receiving 8 Weeks HCV DAA TherapyThe recommended HCV RNA time points for monitoring, which are noted with solid blue circles with surrounding dashed red circles, should be at baseline and again at 12 weeks post treatment.Illustration: David H. Spach, MD
Figure 2. Monitoring of Quantitative HCV RNA Levels in Persons Receiving 8 Weeks HCV DAA TherapyThe recommended HCV RNA time points for monitoring, which are noted with solid blue circles with surrounding dashed red circles, should be at baseline and again at 12 weeks post treatment.Illustration: David H. Spach, MD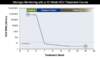 Figure 3. Monitoring of Quantitative HCV RNA Levels in Persons Receiving 12 Weeks HCV DAA TherapyThe recommended HCV RNA time points for monitoring, which are noted with solid blue circles with surrounding dashed red circles, should be at baseline and again at 12 weeks post treatment.Illustration: David H. Spach, MD
Figure 3. Monitoring of Quantitative HCV RNA Levels in Persons Receiving 12 Weeks HCV DAA TherapyThe recommended HCV RNA time points for monitoring, which are noted with solid blue circles with surrounding dashed red circles, should be at baseline and again at 12 weeks post treatment.Illustration: David H. Spach, MD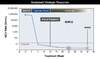 Figure 4. Measurement of Sustained Virologic Response Following HCV TreatmentThis graphic shows common time points for measurement of HCV RNA levels after completion of therapy. The preferred measurement for evaluation of SVR is an HCV RNA level 12 weeks after completing therapy (SVR12). The SVR4 is often obtained in research trials. Some experts evaluate certain patients for SVR24.
Figure 4. Measurement of Sustained Virologic Response Following HCV TreatmentThis graphic shows common time points for measurement of HCV RNA levels after completion of therapy. The preferred measurement for evaluation of SVR is an HCV RNA level 12 weeks after completing therapy (SVR12). The SVR4 is often obtained in research trials. Some experts evaluate certain patients for SVR24. Figure 5. Baseline HBV Parameter in Patients Who Developed HBV Flare During HCV DAA TherapyAbbreviations: DAA = direct-acting antiviralSource: Bersoff-Matcha SJ, Cao K, Jason M, et al. Hepatitis B Virus Reactivation Associated With Direct-Acting Antiviral Therapy for Chronic Hepatitis C Virus: A Review of Cases Reported to the U.S. Food and Drug Administration Adverse Event Reporting System. Ann Intern Med. 2017;166:792-8.
Figure 5. Baseline HBV Parameter in Patients Who Developed HBV Flare During HCV DAA TherapyAbbreviations: DAA = direct-acting antiviralSource: Bersoff-Matcha SJ, Cao K, Jason M, et al. Hepatitis B Virus Reactivation Associated With Direct-Acting Antiviral Therapy for Chronic Hepatitis C Virus: A Review of Cases Reported to the U.S. Food and Drug Administration Adverse Event Reporting System. Ann Intern Med. 2017;166:792-8.Share by e-mail
Check
-On-
Learning
QuestionsThe Check-on-Learning Questions are short and topic related. They are meant to help you stay on track throughout each lesson and check your understanding of key concepts.You must be signed in to customize your interaction with these questions.
- 0%Lesson 2
Since you've received 80% or better on this quiz, you may claim continuing education credit.
You seem to have a popup blocker enabled. If you want to skip this dialog please Always allow popup windows for the online course.
Account Registration Benefits:
- Track your progress on the lessons
- Earn free CNE/CME/CE
- Earn Certificates of Completion
- Access to other free IDEA curricula
Create a free account to get started









